Mechanisms of cancer resistance in long-lived mammals
- PMID: 29622806
- PMCID: PMC6015544
- DOI: 10.1038/s41568-018-0004-9
Mechanisms of cancer resistance in long-lived mammals
Abstract
Cancer researchers have traditionally used the mouse and the rat as staple model organisms. These animals are very short-lived, reproduce rapidly and are highly prone to cancer. They have been very useful for modelling some human cancer types and testing experimental treatments; however, these cancer-prone species offer little for understanding the mechanisms of cancer resistance. Recent technological advances have expanded bestiary research to non-standard model organisms that possess unique traits of very high value to humans, such as cancer resistance and longevity. In recent years, several discoveries have been made in non-standard mammalian species, providing new insights on the natural mechanisms of cancer resistance. These include mechanisms of cancer resistance in the naked mole rat, blind mole rat and elephant. In each of these species, evolution took a different path, leading to novel mechanisms. Many other long-lived mammalian species display cancer resistance, including whales, grey squirrels, microbats, cows and horses. Understanding the molecular mechanisms of cancer resistance in all these species is important and timely, as, ultimately, these mechanisms could be harnessed for the development of human cancer therapies.
Conflict of interest statement
The authors declare no competing interests.
Figures
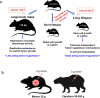
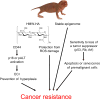
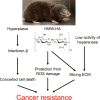


Comment in
-
Insights on cancer resistance in vertebrates: reptiles as a parallel system to mammals.Nat Rev Cancer. 2018 Aug;18(8):525. doi: 10.1038/s41568-018-0033-4. Nat Rev Cancer. 2018. PMID: 29891962 No abstract available.
Similar articles
-
Comparative analysis of long noncoding RNAs in long-lived mammals provides insights into natural cancer-resistance.RNA Biol. 2020 Nov;17(11):1657-1665. doi: 10.1080/15476286.2020.1792116. Epub 2020 Jul 17. RNA Biol. 2020. PMID: 32635806 Free PMC article.
-
Potential Mechanisms for Cancer Resistance in Elephants and Comparative Cellular Response to DNA Damage in Humans.JAMA. 2015 Nov 3;314(17):1850-60. doi: 10.1001/jama.2015.13134. JAMA. 2015. PMID: 26447779 Free PMC article.
-
Chromosome-level Asian elephant genome assembly and comparative genomics of long-lived mammals reveal the common substitutions for cancer resistance.Aging Cell. 2023 Sep;22(9):e13917. doi: 10.1111/acel.13917. Epub 2023 Jul 3. Aging Cell. 2023. PMID: 37395176 Free PMC article.
-
The use of non-traditional models in the study of cancer resistance-the case of the naked mole rat.Oncogene. 2020 Jul;39(28):5083-5097. doi: 10.1038/s41388-020-1355-8. Epub 2020 Jun 13. Oncogene. 2020. PMID: 32535616 Review.
-
The Mystery of Cancer Resistance: A Revelation Within Nature.J Mol Evol. 2023 Apr;91(2):133-155. doi: 10.1007/s00239-023-10092-6. Epub 2023 Jan 24. J Mol Evol. 2023. PMID: 36693985 Review.
Cited by
-
Exploring bat-inspired cyclic tryptophan diketopiperazines as ABCB1 Inhibitors.Commun Chem. 2024 Jul 13;7(1):158. doi: 10.1038/s42004-024-01225-z. Commun Chem. 2024. PMID: 39003409 Free PMC article.
-
Age-dependent expression of cancer-related genes in a long-lived seabird.Evol Appl. 2020 Jun 15;13(7):1708-1718. doi: 10.1111/eva.13024. eCollection 2020 Aug. Evol Appl. 2020. PMID: 32821278 Free PMC article.
-
The role of retrotransposable elements in ageing and age-associated diseases.Nature. 2021 Aug;596(7870):43-53. doi: 10.1038/s41586-021-03542-y. Epub 2021 Aug 4. Nature. 2021. PMID: 34349292 Free PMC article. Review.
-
Spontaneous Disease and Pathology of Naked Mole-Rats.Adv Exp Med Biol. 2021;1319:353-380. doi: 10.1007/978-3-030-65943-1_15. Adv Exp Med Biol. 2021. PMID: 34424525 Review.
-
Ectopic Pregnancy and T-Cell Lymphoma in a Eurasian Red Squirrel (Sciurus vulgaris): Possible Comorbidity and a Comparative Pathology Perspective.Animals (Basel). 2024 Feb 27;14(5):731. doi: 10.3390/ani14050731. Animals (Basel). 2024. PMID: 38473116 Free PMC article.
References
-
- Center for Disease Control and Prevention. United States Cancer Statistics: 1999-2014 Incidence and Mortality Web-based Report
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

