Neutrophil-Hepatic Stellate Cell Interactions Promote Fibrosis in Experimental Steatohepatitis
- PMID: 29552626
- PMCID: PMC5852390
- DOI: 10.1016/j.jcmgh.2018.01.003
Neutrophil-Hepatic Stellate Cell Interactions Promote Fibrosis in Experimental Steatohepatitis
Abstract
Background & aims: Hepatic infiltration of neutrophils is a hallmark of steatohepatitis; however, the role of neutrophils in the progression of steatohepatitis remains unknown.
Methods: A clinically relevant mouse model of steatohepatitis induced by high-fat diet (HFD) plus binge ethanol feeding was used. Liver fibrosis was examined. In vitro cell culture was used to analyze the interaction of hepatic stellate cells (HSCs) and neutrophils.
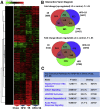
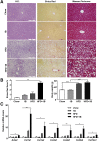
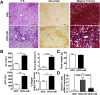
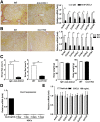
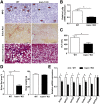
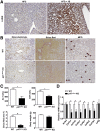
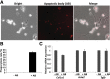
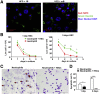
Results: HFD plus one binge ethanol (HFD+1B) feeding induced significant hepatic neutrophil infiltration, liver injury, and fibrosis. HFD plus multiple binges of ethanol (HFD+mB) caused more pronounced liver fibrosis. Microarray analyses showed that the most highly activated signaling pathway in this HFD+1B model was related to liver fibrosis and HSC activation. Blockade of chemokine (C-X-C motif) ligand 1 or intercellular adhesion molecule-1 expression reduced hepatic neutrophil infiltration and ameliorated liver injury and fibrosis. Disruption of the p47phox gene (also called neutrophil cytosolic factor 1), a critical component of reactive oxygen species producing nicotinamide adenine dinucleotide phosphate-oxidase in neutrophils, diminished HFD+1B-induced liver injury and fibrosis. Co-culture of HSCs with neutrophils, but not with neutrophil apoptotic bodies, induced HSC activation and prolonged neutrophil survival. Mechanistic studies showed that activated HSCs produce granulocyte-macrophage colony-stimulating factor and interleukin-15 to prolong the survival of neutrophils, which may serve as a positive forward loop to promote liver damage and fibrosis.
Conclusions: The current data from a mouse model of HFD plus binge ethanol feeding suggest that obesity and binge drinking synergize to promote liver fibrosis, which is partially mediated via the interaction of neutrophils and HSCs. Microarray data in this article have been uploaded to NCBI's Gene Expression Omnibus (GEO accession number: GSE98153).
Keywords: 4-HNE, 4-hydroxynonenal; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Alcohol; CXCL1, chemokine (C-X-C motif) ligand 1; Csf, colony-stimulating factor gene; FBS, fetal bovine serum; Fatty Liver; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; HFD+1B, high-fat diet feeding plus 1 binge of ethanol; HFD+mB, high-fat diet plus multiple binges; HFD, high-fat diet; HSC, hepatic stellate cell; High-Fat Diet; ICAM-1, intercellular adhesion molecule-1; IL, interleukin; Inflammation; KO, knockout; MPO, myeloperoxidase; PCR, polymerase chain reaction; ROS, reactive oxygen species; RT-PCR, reverse-transcription polymerase chain reaction; Reactive Oxygen Species; TUNEL, terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling; WT, wild-type; cDNA, complementary DNA; mRNA, messenger RNA.
Figures

Similar articles
-
Obesity and binge alcohol intake are deadly combination to induce steatohepatitis: A model of high-fat diet and binge ethanol intake.Clin Mol Hepatol. 2020 Oct;26(4):586-594. doi: 10.3350/cmh.2020.0100. Epub 2020 Sep 17. Clin Mol Hepatol. 2020. PMID: 32937687 Free PMC article. Review.
-
Short- or long-term high-fat diet feeding plus acute ethanol binge synergistically induce acute liver injury in mice: an important role for CXCL1.Hepatology. 2015 Oct;62(4):1070-85. doi: 10.1002/hep.27921. Epub 2015 Jul 3. Hepatology. 2015. PMID: 26033752 Free PMC article.
-
Inflammation is independent of steatosis in a murine model of steatohepatitis.Hepatology. 2017 Jul;66(1):108-123. doi: 10.1002/hep.29129. Epub 2017 May 18. Hepatology. 2017. PMID: 28220523 Free PMC article.
-
Interleukin-22 Ameliorates Neutrophil-Driven Nonalcoholic Steatohepatitis Through Multiple Targets.Hepatology. 2020 Aug;72(2):412-429. doi: 10.1002/hep.31031. Epub 2020 Mar 16. Hepatology. 2020. PMID: 31705800 Free PMC article.
-
Decoding cell death signals in liver inflammation.J Hepatol. 2013 Sep;59(3):583-94. doi: 10.1016/j.jhep.2013.03.033. Epub 2013 Apr 6. J Hepatol. 2013. PMID: 23567086 Review.
Cited by
-
A Review of CXCL1 in Cardiac Fibrosis.Front Cardiovasc Med. 2021 Apr 28;8:674498. doi: 10.3389/fcvm.2021.674498. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 33996954 Free PMC article.
-
The association of systemic inflammatory biomarkers with non-alcoholic fatty liver disease: a large population-based cross-sectional study.Prev Med Rep. 2023 Dec 7;37:102536. doi: 10.1016/j.pmedr.2023.102536. eCollection 2024 Jan. Prev Med Rep. 2023. PMID: 38186662 Free PMC article.
-
Cellular communication network factor 1-stimulated liver macrophage efferocytosis drives hepatic stellate cell activation and liver fibrosis.Hepatol Commun. 2022 Oct;6(10):2798-2811. doi: 10.1002/hep4.2057. Epub 2022 Aug 5. Hepatol Commun. 2022. PMID: 35929736 Free PMC article.
-
Obesity in acute alcoholic hepatitis increases morbidity and mortality.EBioMedicine. 2019 Jul;45:511-518. doi: 10.1016/j.ebiom.2019.03.046. Epub 2019 Jul 3. EBioMedicine. 2019. PMID: 31278069 Free PMC article.
-
Targeting SYK signaling in myeloid cells protects against liver fibrosis and hepatocarcinogenesis.Oncogene. 2019 Jun;38(23):4512-4526. doi: 10.1038/s41388-019-0734-5. Epub 2019 Feb 11. Oncogene. 2019. PMID: 30742098
References
-
- Rinella M.E. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–2273. - PubMed
-
- Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

