A novel approach to adenine-induced chronic kidney disease associated anemia in rodents
- PMID: 29415057
- PMCID: PMC5802942
- DOI: 10.1371/journal.pone.0192531
A novel approach to adenine-induced chronic kidney disease associated anemia in rodents
Abstract
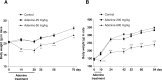
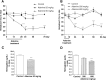
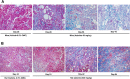
To date, good experimental animal models of renal anemia are not available. Therefore, the purpose of this study was to establish a novel approach to induce chronic kidney disease (CKD) with severe anemia by oral administration of adenine in rodents. Adenine was administered to 6-week-old male C57BL/6 mice (25 and 50 mg/kg body weight) by oral gavage daily for 28 days. Serum creatinine and BUN as well as hematocrit, hemoglobin (Hb) and plasma erythropoietin (EPO) levels were monitored to assess renal function and anemia, respectively. Adenine at 25 mg/kg for 28 days slightly increased plasma creatinine levels, but did not induce anemia. In contrast, 50 mg/kg of adenine daily for 28 days showed severe renal dysfunction (plasma creatinine 1.9 ± 0.10 mg/dL) and anemia (hematocrit 36.5 ± 1.0% and EPO 28 ± 2.4 pg/mL) as compared with vehicle-treated mice (0.4 ± 0.02 mg/dL, 49.6 ± 1.6% and 61 ± 4.0 pg/mL, respectively). At the end of experiment, level of Hb also significantly reduced in 50 mg/kg adenine administration group. Remarkable histological changes of kidney tissues characterized by interstitial fibrosis and cystic appearance in tubules were observed in 50 mg/kg of adenine treatment group. These results have demonstrated that oral dosing with adenine at 50 mg/kg for 28 days is suitable to induce a stable anemia associated with CKD in mice.
Conflict of interest statement
Figures




Similar articles
-
Failure to confirm a sodium-glucose cotransporter 2 inhibitor-induced hematopoietic effect in non-diabetic rats with renal anemia.J Diabetes Investig. 2020 Jul;11(4):834-843. doi: 10.1111/jdi.13205. Epub 2020 Feb 5. J Diabetes Investig. 2020. PMID: 31880858 Free PMC article.
-
Effects of erythropoietin-gene electrotransfer in rats with adenine-induced renal failure.Am J Nephrol. 2003 Sep-Oct;23(5):315-23. doi: 10.1159/000072913. Epub 2003 Aug 12. Am J Nephrol. 2003. PMID: 12915775
-
Progression of anemia and its relationship with renal function, blood pressure, and erythropoietin in rats with chronic kidney disease.Vet Clin Pathol. 2015 Sep;44(3):342-54. doi: 10.1111/vcp.12276. Epub 2015 Aug 20. Vet Clin Pathol. 2015. PMID: 26292198
-
[Erythropoietin-beta in the treatment of anemia in patients with chronic renal insufficiency].Med Pregl. 2001 May-Jun;54(5-6):235-40. Med Pregl. 2001. PMID: 11759218 Review. Croatian.
-
The interaction between heart failure and other heart diseases, renal failure, and anemia.Semin Nephrol. 2006 Jul;26(4):296-306. doi: 10.1016/j.semnephrol.2006.05.006. Semin Nephrol. 2006. PMID: 16949468 Review.
Cited by
-
Common Dietary Modifications in Preclinical Models to Study Skeletal Health.Front Endocrinol (Lausanne). 2022 Jul 14;13:932343. doi: 10.3389/fendo.2022.932343. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35909523 Free PMC article. Review.
-
Adenine-Induced Nephropathy Reduces Atherosclerosis in ApoE Knockout Mice.Biomolecules. 2022 Aug 19;12(8):1147. doi: 10.3390/biom12081147. Biomolecules. 2022. PMID: 36009040 Free PMC article.
-
Adenine's impact on mice's gut and kidney varies with the dosage administered and relates to intestinal microorganisms and enzyme activities.3 Biotech. 2024 Mar;14(3):88. doi: 10.1007/s13205-024-03959-y. Epub 2024 Feb 22. 3 Biotech. 2024. PMID: 38406640
-
Untargeted metabolomics reveals gender- and age- independent metabolic changes of type 1 diabetes in Chinese children.Front Endocrinol (Lausanne). 2022 Dec 22;13:1037289. doi: 10.3389/fendo.2022.1037289. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36619558 Free PMC article.
-
Failure to confirm a sodium-glucose cotransporter 2 inhibitor-induced hematopoietic effect in non-diabetic rats with renal anemia.J Diabetes Investig. 2020 Jul;11(4):834-843. doi: 10.1111/jdi.13205. Epub 2020 Feb 5. J Diabetes Investig. 2020. PMID: 31880858 Free PMC article.
References
-
- Kdoqi, National Kidney F. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2006;47(5 Suppl 3):S11–145. doi: 10.1053/j.ajkd.2006.03.010 . - DOI - PubMed
-
- Wang X, Qiu M, Li J, Wang H, Qi J, Wang G, et al. Impacts of anemia on 3-year ischemic events in patients undergoing percutaneous coronary intervention: a propensity-matched study. Journal of thoracic disease. 2015;7(11):1951–9. doi: 10.3978/j.issn.2072-1439.2015.10.66 ; PubMed Central PMCID: PMC4669295. - DOI - PMC - PubMed
-
- Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, et al. Correction of anemia with epoetin alfa in chronic kidney disease. The New England journal of medicine. 2006;355(20):2085–98. doi: 10.1056/NEJMoa065485 . - DOI - PubMed
-
- Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. The New England journal of medicine. 2009;361(21):2019–32. doi: 10.1056/NEJMoa0907845 . - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

