Thrombin@Fe3O4 nanoparticles for use as a hemostatic agent in internal bleeding
- PMID: 29321571
- PMCID: PMC5762673
- DOI: 10.1038/s41598-017-18665-4
Thrombin@Fe3O4 nanoparticles for use as a hemostatic agent in internal bleeding
Erratum in
-
Author Correction: Thrombin@Fe3O4 nanoparticles for use as a hemostatic agent in internal bleeding.Sci Rep. 2024 Feb 27;14(1):4731. doi: 10.1038/s41598-024-53918-z. Sci Rep. 2024. PMID: 38413674 Free PMC article. No abstract available.
Abstract
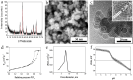
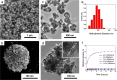
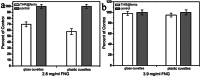
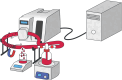
Bleeding remains one of the main causes of premature mortality at present, with internal bleeding being the most dangerous case. In this paper, magnetic hemostatic nanoparticles are shown for the first time to assist in minimally invasive treatment of internal bleeding, implying the introduction directly into the circulatory system followed by localization in the bleeding zone due to the application of an external magnetic field. Nanoparticles were produced by entrapping human thrombin (THR) into a sol-gel derived magnetite matrix followed by grinding to sizes below 200 nm and subsequent colloidization. Prepared colloids show protrombotic activity and cause plasma coagulation in in vitro experiments. We also show here using a model blood vessel that the THR@ferria composite does not cause systematic thrombosis due to low activity, but being concentrated by an external magnetic field with simultaneous fibrinogen injection accelerates local hemostasis and stops the bleeding. For instance, a model vessel system with circulating blood at the puncture of the vessel wall and the application of a permanent magnetic field yielded a hemostasis time by a factor of 6.5 shorter than that observed for the control sample. Biocompatibility of composites was tested on HELF and HeLa cells and revealed no toxic effects.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


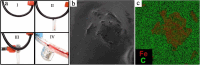
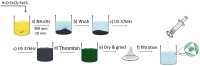
Similar articles
-
Nanocomplexation of thrombin with cationic amylose derivative for improved stability and hemostatic efficacy.Int J Nanomedicine. 2015 Jan 29;10:939-47. doi: 10.2147/IJN.S72553. eCollection 2015. Int J Nanomedicine. 2015. PMID: 25673989 Free PMC article.
-
A facile way to fabricate a thrombin immobilized composite sponge with dual hemostatic effects for acute hemorrhage control.Biomater Adv. 2025 Jan;166:214037. doi: 10.1016/j.bioadv.2024.214037. Epub 2024 Sep 8. Biomater Adv. 2025. PMID: 39276658
-
Potential role of recombinant factor VIIa as a hemostatic agent.Clin Adv Hematol Oncol. 2003 Feb;1(2):112-9. Clin Adv Hematol Oncol. 2003. PMID: 16224390 Review.
-
Slounase, a Batroxobin Containing Activated Factor X Effectively Enhances Hemostatic Clot Formation and Reducing Bleeding in Hypocoagulant Conditions in Mice.Clin Appl Thromb Hemost. 2021 Jan-Dec;27:10760296211018510. doi: 10.1177/10760296211018510. Clin Appl Thromb Hemost. 2021. PMID: 34047195 Free PMC article.
-
External Bleeding and Advanced Biomacromolecules for Hemostasis.Biomacromolecules. 2024 Nov 11;25(11):6936-6966. doi: 10.1021/acs.biomac.4c00952. Epub 2024 Oct 28. Biomacromolecules. 2024. PMID: 39463174 Review.
Cited by
-
Improved hemostatic effects by Fe3+ modified biomimetic PLLA cotton-like mat via sodium alginate grafted with dopamine.Bioact Mater. 2021 Jan 25;6(8):2346-2359. doi: 10.1016/j.bioactmat.2021.01.002. eCollection 2021 Aug. Bioact Mater. 2021. PMID: 33553820 Free PMC article.
-
Test-System for Bacteria Sensing Based on Peroxidase-Like Activity of Inkjet-Printed Magnetite Nanoparticles.Nanomaterials (Basel). 2020 Feb 12;10(2):313. doi: 10.3390/nano10020313. Nanomaterials (Basel). 2020. PMID: 32059377 Free PMC article.
-
Vancomycin and nisin-modified magnetic Fe3O4@SiO2 nanostructures coated with chitosan to enhance antibacterial efficiency against methicillin resistant Staphylococcus aureus (MRSA) infection in a murine superficial wound model.BMC Chem. 2024 Feb 23;18(1):43. doi: 10.1186/s13065-024-01129-y. BMC Chem. 2024. PMID: 38395982 Free PMC article.
-
Effect of hydroxyethyl cellulose soluble hemostatic gauze on hemostasis in facial contouring surgery.Medicine (Baltimore). 2021 May 14;100(19):e25847. doi: 10.1097/MD.0000000000025847. Medicine (Baltimore). 2021. PMID: 34106626 Free PMC article. Clinical Trial.
-
Nanomaterial Shape Influence on Cell Behavior.Int J Mol Sci. 2021 May 17;22(10):5266. doi: 10.3390/ijms22105266. Int J Mol Sci. 2021. PMID: 34067696 Free PMC article. Review.
References
-
- Whang HS, Kirsch W, Zhu YH, Yang CZ, Hudson SM. Hemostatic agents derived from chitin and chitosan. Journal of Macromolecular Science, Part C: Polymer Reviews. 2005;45:309–323. doi: 10.1080/15321790500304122. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

