Induction of autophagy and autophagy-dependent apoptosis in diffuse large B-cell lymphoma by a new antimalarial artemisinin derivative, SM1044
- PMID: 29277967
- PMCID: PMC5806110
- DOI: 10.1002/cam4.1276
Induction of autophagy and autophagy-dependent apoptosis in diffuse large B-cell lymphoma by a new antimalarial artemisinin derivative, SM1044
Abstract
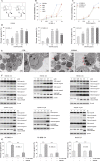
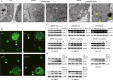
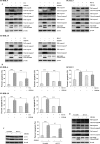
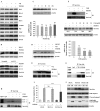
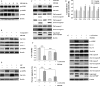
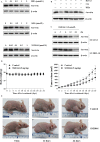
Diffuse large B-cell lymphoma (DLBCL) is the most common form of non-Hodgkin's lymphoma. R-CHOP is currently the standard therapy for DLBCL, but the prognosis of refractory or recurrent patients remains poor. In this study, we synthesized a new water-soluble antimalarial drug artemisinin derivative, SM1044. The treatment of DLBCL cell lines with SM1044 induces autophagy-dependent apoptosis, which is directed by an accelerated degradation of the antiapoptosis protein Survivin, via its acetylation-dependent interaction with the autophagy-related protein LC3-II. Additionally, SM1044 also stimulates the de novo synthesis of ceramide, which in turn activates the CaMKK2-AMPK-ULK1 axis, leading to the initiation of autophagy. Our findings not only elucidate the mechanism of autophagy-dependent apoptosis in DLBCL cells, but also suggest that SM1044 is a promising therapeutic molecule for the treatment of DLBCL, along with R-CHOP regimen.
Keywords: DLBCL; Apoptosis; Survivin; artemisinin derivative; autophagy.
© 2017 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures
Similar articles
-
[A new artemisinin derivative SM1044 induces apoptosis of Kasumi-1 cells and its mechanism].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2011 Jun;19(3):607-11. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2011. PMID: 21729533 Chinese.
-
The novel LSD1 inhibitor ZY0511 suppresses diffuse large B-cell lymphoma proliferation by inducing apoptosis and autophagy.Med Oncol. 2021 Sep 7;38(10):124. doi: 10.1007/s12032-021-01572-0. Med Oncol. 2021. PMID: 34491469 Free PMC article.
-
Lymphoma and myeloma cells are highly sensitive to growth arrest and apoptosis induced by artesunate.Eur J Haematol. 2013 Oct;91(4):339-46. doi: 10.1111/ejh.12176. Epub 2013 Aug 20. Eur J Haematol. 2013. PMID: 23869695
-
Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia.Drugs. 2003;63(8):803-43. doi: 10.2165/00003495-200363080-00005. Drugs. 2003. PMID: 12662126 Review.
-
[Progress of study on survivin in diffuse large B-cell lymphoma--review].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2008 Dec;16(6):1487-90. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2008. PMID: 19099670 Review. Chinese.
Cited by
-
Will AMPK be a potential therapeutic target for hepatocellular carcinoma?Am J Cancer Res. 2024 Jul 15;14(7):3241-3258. doi: 10.62347/YAVK1315. eCollection 2024. Am J Cancer Res. 2024. PMID: 39113872 Free PMC article. Review.
-
Prediction significance of autophagy-related genes in survival probability and drug resistance in diffuse large B-cell lymphoma.Aging (Albany NY). 2024 Jan 17;16(2):1049-1076. doi: 10.18632/aging.205282. Epub 2024 Jan 17. Aging (Albany NY). 2024. PMID: 38240686 Free PMC article.
-
Targeting YTHDF2 inhibits tumorigenesis of diffuse large B-cell lymphoma through ACER2-mediated ceramide catabolism.J Adv Res. 2024 Sep;63:17-33. doi: 10.1016/j.jare.2023.10.010. Epub 2023 Oct 19. J Adv Res. 2024. PMID: 37865189 Free PMC article.
-
Long non-coding RNA mitophagy and ALK-negative anaplastic lymphoma-associated transcript: a novel regulator of mitophagy in T-cell lymphoma.Haematologica. 2023 Dec 1;108(12):3333-3346. doi: 10.3324/haematol.2022.282552. Haematologica. 2023. PMID: 37381763 Free PMC article.
-
Altered pathways and targeted therapy in double hit lymphoma.J Hematol Oncol. 2022 Mar 18;15(1):26. doi: 10.1186/s13045-022-01249-9. J Hematol Oncol. 2022. PMID: 35303910 Free PMC article. Review.
References
-
- Chaganti, S. , Illidge T., Barrington S., McKay P., Linton K., Cwynarski K., et al. 2016. Guidelines for the management of diffuse large B‐cell lymphoma. Br. J. Haematol. 174:43–56. - PubMed
-
- Friedberg, J. W. 2011. Relapsed/refractory diffuse large B‐cell lymphoma. Hematol. Am. Soc. Hematol. Edu. Program 2011:498–505. - PubMed
-
- Perry, A. M. , Mitrovic Z., and Chan W. C.. 2012. Biological prognostic markers in diffuse large B‐cell lymphoma. Cancer Control 19:214–226. - PubMed
-
- Liu, Z. , Xu‐Monette Z. Y., Cao X., Manyam G. C., Wang X., Tzankov A., et al. 2015. Prognostic and biological significance of Survivin expression in patients with diffuse large B‐cell lymphoma treated with rituximab‐CHOP therapy. Modern Pathol. 28: 1297–1314. - PubMed
-
- Kawasaki, H. , Altieri D. C., Lu C. D., Toyoda M., Tenjo T., and Tanigawa N.. 1998. Inhibition of apoptosis by Survivin predicts shorter survival rates in colorectal cancer. Can. Res. 58:5071–5074. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

