Endocytosis regulates TDP-43 toxicity and turnover
- PMID: 29233983
- PMCID: PMC5727062
- DOI: 10.1038/s41467-017-02017-x
Endocytosis regulates TDP-43 toxicity and turnover
Abstract
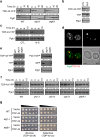
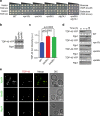
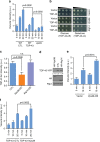
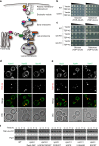
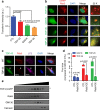
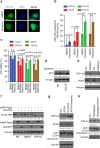
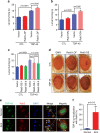
Amyotrophic lateral sclerosis (ALS) is a fatal motor neuron degenerative disease. ALS-affected motor neurons exhibit aberrant localization of a nuclear RNA binding protein, TDP-43, into cytoplasmic aggregates, which contributes to pathology via unclear mechanisms. Here, we demonstrate that TDP-43 turnover and toxicity depend in part upon the endocytosis pathway. TDP-43 inhibits endocytosis, and co-localizes strongly with endocytic proteins, including in ALS patient tissue. Impairing endocytosis increases TDP-43 toxicity, aggregation, and protein levels, whereas enhancing endocytosis reverses these phenotypes. Locomotor dysfunction in a TDP-43 ALS fly model is also exacerbated and suppressed by impairment and enhancement of endocytic function, respectively. Thus, endocytosis dysfunction may be an underlying cause of ALS pathology.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Cdc48/VCP and Endocytosis Regulate TDP-43 and FUS Toxicity and Turnover.Mol Cell Biol. 2020 Jan 30;40(4):e00256-19. doi: 10.1128/MCB.00256-19. Print 2020 Jan 30. Mol Cell Biol. 2020. PMID: 31767634 Free PMC article.
-
Motor neurons and glia exhibit specific individualized responses to TDP-43 expression in a Drosophila model of amyotrophic lateral sclerosis.Dis Model Mech. 2013 May;6(3):721-33. doi: 10.1242/dmm.010710. Epub 2013 Feb 1. Dis Model Mech. 2013. PMID: 23471911 Free PMC article.
-
Increased cytoplasmic TARDBP mRNA in affected spinal motor neurons in ALS caused by abnormal autoregulation of TDP-43.Nucleic Acids Res. 2016 Jul 8;44(12):5820-36. doi: 10.1093/nar/gkw499. Epub 2016 Jun 2. Nucleic Acids Res. 2016. PMID: 27257061 Free PMC article.
-
The molecular link between inefficient GluA2 Q/R site-RNA editing and TDP-43 pathology in motor neurons of sporadic amyotrophic lateral sclerosis patients.Brain Res. 2014 Oct 10;1584:28-38. doi: 10.1016/j.brainres.2013.12.011. Epub 2013 Dec 16. Brain Res. 2014. PMID: 24355598 Review.
-
The debated toxic role of aggregated TDP-43 in amyotrophic lateral sclerosis: a resolution in sight?Brain. 2019 May 1;142(5):1176-1194. doi: 10.1093/brain/awz078. Brain. 2019. PMID: 30938443 Free PMC article. Review.
Cited by
-
Emerging Therapies and Novel Targets for TDP-43 Proteinopathy in ALS/FTD.Neurotherapeutics. 2022 Jul;19(4):1061-1084. doi: 10.1007/s13311-022-01260-5. Epub 2022 Jul 5. Neurotherapeutics. 2022. PMID: 35790708 Free PMC article. Review.
-
Emerging Trends in the Field of Inflammation and Proteinopathy in ALS/FTD Spectrum Disorder.Biomedicines. 2023 May 31;11(6):1599. doi: 10.3390/biomedicines11061599. Biomedicines. 2023. PMID: 37371694 Free PMC article. Review.
-
Trends in Understanding the Pathological Roles of TDP-43 and FUS Proteins.Adv Exp Med Biol. 2021;1281:243-267. doi: 10.1007/978-3-030-51140-1_15. Adv Exp Med Biol. 2021. PMID: 33433879
-
Neurotoxic microglia promote TDP-43 proteinopathy in progranulin deficiency.Nature. 2020 Dec;588(7838):459-465. doi: 10.1038/s41586-020-2709-7. Epub 2020 Aug 31. Nature. 2020. PMID: 32866962 Free PMC article.
-
Insights of Endocytosis Signaling in Health and Disease.Int J Mol Sci. 2023 Feb 3;24(3):2971. doi: 10.3390/ijms24032971. Int J Mol Sci. 2023. PMID: 36769293 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

