Local redox environment beneath biological membranes probed by palmitoylated-roGFP
- PMID: 29179107
- PMCID: PMC5704182
- DOI: 10.1016/j.redox.2017.11.015
Local redox environment beneath biological membranes probed by palmitoylated-roGFP
Abstract
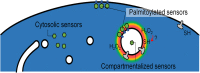
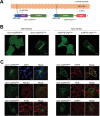
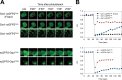
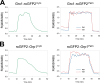
Production of reactive oxygen species (ROS) and consequent glutathione oxidation are associated with various physiological processes and diseases, including cell differentiation, senescence, and inflammation. GFP-based redox sensors provide a straight-forward approach to monitor ROS levels and glutathione oxidation within a living cell at the subcellular resolution. We utilized palmitoylated versions of cytosolic glutathione and hydrogen peroxide sensors (Grx1-roGFP2 and roGFP2-Orp1, respectively) and demonstrated a unique redox environment near biological membranes. In HeLa cells, cytosolic glutathione was practically completely reduced (EGSH/GSSG = - 333mV) and hydrogen peroxide level was under the detectable range. In contrast, the cytoplasmic milieu near membranes of intracellular vesicles exhibited significant glutathione oxidation (EGSH/GSSG > - 256mV) and relatively high H2O2 production, which was not observed for the plasma membrane. These vesicles colocalized with internalized EGFR, suggesting that H2O2 production and glutathione oxidation are characteristics of cytoplasmic surfaces of the endocytosed vesicles. The results visually illustrate local redox heterogeneity within the cytosol for the first time.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
Similar articles
-
[Analyzing the Redox Status of Intracellular Glutathione and Its Application to an Intestinal Bowel Disease Model].Yakugaku Zasshi. 2019;139(12):1523-1530. doi: 10.1248/yakushi.19-00184. Yakugaku Zasshi. 2019. PMID: 31787639 Review. Japanese.
-
Live Monitoring of ROS-Induced Cytosolic Redox Changes with roGFP2-Based Sensors in Plants.Methods Mol Biol. 2022;2526:65-85. doi: 10.1007/978-1-0716-2469-2_5. Methods Mol Biol. 2022. PMID: 35657512
-
Measuring Mitochondrial Hydrogen Peroxide Levels and Glutathione Redox Equilibrium in Drosophila Neuron Subtypes Using Redox-Sensitive Fluorophores and 3D Imaging.Methods Mol Biol. 2021;2276:113-127. doi: 10.1007/978-1-0716-1266-8_8. Methods Mol Biol. 2021. PMID: 34060036
-
Genetically Encoded Biosensors to Monitor Intracellular Reactive Oxygen and Nitrogen Species and Glutathione Redox Potential in Skeletal Muscle Cells.Int J Mol Sci. 2021 Oct 8;22(19):10876. doi: 10.3390/ijms221910876. Int J Mol Sci. 2021. PMID: 34639217 Free PMC article.
-
Mechanisms and Applications of Redox-Sensitive Green Fluorescent Protein-Based Hydrogen Peroxide Probes.Antioxid Redox Signal. 2018 Aug 20;29(6):552-568. doi: 10.1089/ars.2017.7449. Epub 2018 Jan 10. Antioxid Redox Signal. 2018. PMID: 29160083 Review.
Cited by
-
Cross talk between redox signalling and metabolic activity of osteoblasts and fibroblasts in the presence of hydroxyapatite-based biomaterials influences bone regeneration.J Appl Biomed. 2019 Jun;17(2):125-135. doi: 10.32725/jab.2019.004. Epub 2019 Apr 15. J Appl Biomed. 2019. PMID: 34907734
-
Irisin activates Opa1-induced mitophagy to protect cardiomyocytes against apoptosis following myocardial infarction.Aging (Albany NY). 2020 Mar 10;12(5):4474-4488. doi: 10.18632/aging.102899. Epub 2020 Mar 10. Aging (Albany NY). 2020. PMID: 32155590 Free PMC article.
-
Mechanotransduction in talin through the interaction of the R8 domain with DLC1.PLoS Biol. 2018 Jul 20;16(7):e2005599. doi: 10.1371/journal.pbio.2005599. eCollection 2018 Jul. PLoS Biol. 2018. PMID: 30028837 Free PMC article.
-
Mammalian STE20-like Kinase 1 Knockdown Attenuates TNFα-Mediated Neurodegenerative Disease by Repressing the JNK Pathway and Mitochondrial Stress.Neurochem Res. 2019 Jul;44(7):1653-1664. doi: 10.1007/s11064-019-02791-8. Epub 2019 Apr 4. Neurochem Res. 2019. PMID: 30949935
-
ATP13A2 modifies mitochondrial localization of overexpressed TOM20 to autolysosomal pathway.PLoS One. 2022 Nov 29;17(11):e0276823. doi: 10.1371/journal.pone.0276823. eCollection 2022. PLoS One. 2022. PMID: 36445873 Free PMC article.
References
-
- Finkel T., Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. - PubMed
-
- Nathan C., Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. - PubMed
-
- Schafer F.Q., Buettner G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001;30:1191–1212. - PubMed
-
- Bonekamp N.A., Völkl A., Fahimi H.D., Schrader M. Reactive oxygen species and peroxisomes: struggling for balance. BioFactors. 2009;35:346–355. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

