HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study
- PMID: 29112956
- PMCID: PMC5675377
- DOI: 10.1371/journal.pmed.1002417
HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study
Abstract
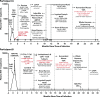
Background: It is unknown if extremely early initiation of antiretroviral therapy (ART) may lead to long-term ART-free HIV remission or cure. As a result, we studied 2 individuals recruited from a pre-exposure prophylaxis (PrEP) program who started prophylactic ART an estimated 10 days (Participant A; 54-year-old male) and 12 days (Participant B; 31-year-old male) after infection with peak plasma HIV RNA of 220 copies/mL and 3,343 copies/mL, respectively. Extensive testing of blood and tissue for HIV persistence was performed, and PrEP Participant A underwent analytical treatment interruption (ATI) following 32 weeks of continuous ART.
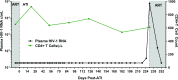
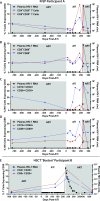
Methods and findings: Colorectal and lymph node tissues, bone marrow, cerebral spinal fluid (CSF), plasma, and very large numbers of peripheral blood mononuclear cells (PBMCs) were obtained longitudinally from both participants and were studied for HIV persistence in several laboratories using molecular and culture-based detection methods, including a murine viral outgrowth assay (mVOA). Both participants initiated PrEP with tenofovir/emtricitabine during very early Fiebig stage I (detectable plasma HIV-1 RNA, antibody negative) followed by 4-drug ART intensification. Following peak viral loads, both participants experienced full suppression of HIV-1 plasma viremia. Over the following 2 years, no further HIV could be detected in blood or tissue from PrEP Participant A despite extensive sampling from ileum, rectum, lymph nodes, bone marrow, CSF, circulating CD4+ T cell subsets, and plasma. No HIV was detected from tissues obtained from PrEP Participant B, but low-level HIV RNA or DNA was intermittently detected from various CD4+ T cell subsets. Over 500 million CD4+ T cells were assayed from both participants in a humanized mouse outgrowth assay. Three of 8 mice infused with CD4+ T cells from PrEP Participant B developed viremia (50 million input cells/surviving mouse), but only 1 of 10 mice infused with CD4+ T cells from PrEP Participant A (53 million input cells/mouse) experienced very low level viremia (201 copies/mL); sequence confirmation was unsuccessful. PrEP Participant A stopped ART and remained aviremic for 7.4 months, rebounding with HIV RNA of 36 copies/mL that rose to 59,805 copies/mL 6 days later. ART was restarted promptly. Rebound plasma HIV sequences were identical to those obtained during acute infection by single-genome sequencing. Mathematical modeling predicted that the latent reservoir size was approximately 200 cells prior to ATI and that only around 1% of individuals with a similar HIV burden may achieve lifelong ART-free remission. Furthermore, we observed that lymphocytes expressing the tumor marker CD30 increased in frequency weeks to months prior to detectable HIV-1 RNA in plasma. This study was limited by the small sample size, which was a result of the rarity of individuals presenting during hyperacute infection.
Conclusions: We report HIV relapse despite initiation of ART at one of the earliest stages of acute HIV infection possible. Near complete or complete loss of detectable HIV in blood and tissues did not lead to indefinite ART-free HIV remission. However, the small numbers of latently infected cells in individuals treated during hyperacute infection may be associated with prolonged ART-free remission.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: AYL has led trials in which Gilead has donated study drug. SEC was the protocol co-chair an a site principle investigator of the US PrEP demonstration project for which Gilead donated study drug. JWM has share options in C0-Crystal, Inc; is on the scientific advisory board for Gilead Sciences; and has received research funding from Gilead Sciences, Janssen Pharmaceuticals, and Abbott Molecular. SGD declares the research group has received external funding to support our research from ViiV, Gilead, and Merck. He is also a guest editor for the
Figures
Similar articles
-
Cells producing residual viremia during antiretroviral treatment appear to contribute to rebound viremia following interruption of treatment.PLoS Pathog. 2020 Aug 25;16(8):e1008791. doi: 10.1371/journal.ppat.1008791. eCollection 2020 Aug. PLoS Pathog. 2020. PMID: 32841299 Free PMC article.
-
Origin of Rebound Plasma HIV Includes Cells with Identical Proviruses That Are Transcriptionally Active before Stopping of Antiretroviral Therapy.J Virol. 2015 Nov 18;90(3):1369-76. doi: 10.1128/JVI.02139-15. Print 2016 Feb 1. J Virol. 2015. PMID: 26581989 Free PMC article.
-
Circulating CD30+CD4+ T Cells Increase Before Human Immunodeficiency Virus Rebound After Analytical Antiretroviral Treatment Interruption.J Infect Dis. 2020 Mar 16;221(7):1146-1155. doi: 10.1093/infdis/jiz572. J Infect Dis. 2020. PMID: 31677350 Free PMC article.
-
Interventions during Early Infection: Opening a Window for an HIV Cure?Viruses. 2024 Oct 9;16(10):1588. doi: 10.3390/v16101588. Viruses. 2024. PMID: 39459922 Free PMC article. Review.
-
Early antiretroviral therapy in HIV-infected infants: can it lead to HIV remission?Lancet HIV. 2018 May;5(5):e250-e258. doi: 10.1016/S2352-3018(18)30012-2. Epub 2018 May 1. Lancet HIV. 2018. PMID: 29739699 Free PMC article. Review.
Cited by
-
Impact of alemtuzumab-mediated lymphocyte depletion on SIV reservoir establishment and persistence.PLoS Pathog. 2024 Aug 22;20(8):e1012496. doi: 10.1371/journal.ppat.1012496. eCollection 2024 Aug. PLoS Pathog. 2024. PMID: 39173097 Free PMC article.
-
Autologous dendritic cell vaccination against HIV-1 induces changes in natural killer cell phenotype and functionality.NPJ Vaccines. 2023 Mar 2;8(1):29. doi: 10.1038/s41541-023-00631-z. NPJ Vaccines. 2023. PMID: 36864042 Free PMC article.
-
Prevention of SIVmac251 reservoir seeding in rhesus monkeys by early antiretroviral therapy.Nat Commun. 2018 Dec 21;9(1):5429. doi: 10.1038/s41467-018-07881-9. Nat Commun. 2018. PMID: 30575753 Free PMC article.
-
Early treatment regimens achieve sustained virologic remission in infant macaques infected with SIV at birth.Nat Commun. 2022 Aug 16;13(1):4823. doi: 10.1038/s41467-022-32554-z. Nat Commun. 2022. PMID: 35973985 Free PMC article.
-
Viral and Host Biomarkers of HIV Remission Post Treatment Interruption.Curr HIV/AIDS Rep. 2022 Jun;19(3):217-233. doi: 10.1007/s11904-022-00607-z. Epub 2022 Apr 19. Curr HIV/AIDS Rep. 2022. PMID: 35438384 Review.
References
-
- Deeks SG, Lewin SR, Ross AL, Ananworanich J, Benkirane M, Cannon P, et al. International AIDS Society global scientific strategy: towards an HIV cure 2016. Nat Med. 2016. August;22(8):839–50. doi: 10.1038/nm.4108 - DOI - PMC - PubMed
-
- Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4(+) T cells. Nat Med. 2003. June;9(6):727–8. doi: 10.1038/nm880 - DOI - PubMed
-
- Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009. March 6;323(5919):1304–7. doi: 10.1126/science.1165706 - DOI - PubMed
-
- Ananworanich J, Puthanakit T, Suntarattiwong P, Chokephaibulkit K, Kerr SJ, Fromentin R, et al. Reduced markers of HIV persistence and restricted HIV-specific immune responses after early antiretroviral therapy in children. AIDS. 2014. April 24;28(7):1015–20. doi: 10.1097/QAD.0000000000000178 - DOI - PubMed
-
- Schuetz A, Deleage C, Sereti I, Rerknimitr R, Phanuphak N, Phuang-Ngern Y, et al. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog. 2014. December;10(12):e1004543 doi: 10.1371/journal.ppat.1004543 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UM1 AI126620/AI/NIAID NIH HHS/United States
- R33 AI116205/AI/NIAID NIH HHS/United States
- IK2 CX000520/CX/CSRD VA/United States
- K01 OD018244/OD/NIH HHS/United States
- P30 AI094189/AI/NIAID NIH HHS/United States
- UM1 AI126619/AI/NIAID NIH HHS/United States
- U19 AI096109/AI/NIAID NIH HHS/United States
- R01 DK108349/DK/NIDDK NIH HHS/United States
- R21 AI116205/AI/NIAID NIH HHS/United States
- R01 AI132128/AI/NIAID NIH HHS/United States
- U19 AI096113/AI/NIAID NIH HHS/United States
- R01 NS094067/NS/NINDS NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- R01 AI122862/AI/NIAID NIH HHS/United States
- R01 AI120024/AI/NIAID NIH HHS/United States
- K23 AI098480/AI/NIAID NIH HHS/United States
- P30 AI036214/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

