[10]-gingerol induces apoptosis and inhibits metastatic dissemination of triple negative breast cancer in vivo
- PMID: 29069785
- PMCID: PMC5641128
- DOI: 10.18632/oncotarget.20139
[10]-gingerol induces apoptosis and inhibits metastatic dissemination of triple negative breast cancer in vivo
Abstract
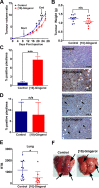
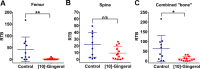
There is increasing interest in the use of non-toxic natural products for the treatment of various pathologies, including cancer. In particular, biologically active constituents of the ginger oleoresin (Zingiber officinale Roscoe) have been shown to mediate anti-tumour activity and to contribute to the anti-inflammatory, antioxidant, antimicrobial, and antiemetic properties of ginger. Here we report on the inhibitory properties of [10]-gingerol against metastatic triple negative breast cancer (TNBC) in vitro and in vivo. We show that [10]-gingerol concentration-dependently induces apoptotic death in mouse and human TNBC cell lines in vitro. In addition, [10]-gingerol is well tolerated in vivo, induces a marked increase in caspase-3 activation and inhibits orthotopic tumour growth in a syngeneic mouse model of spontaneous breast cancer metastasis. Importantly, using both spontaneous and experimental metastasis assays, we show for the first time that [10]-gingerol significantly inhibits metastasis to multiple organs including lung, bone and brain. Remarkably, inhibition of brain metastasis was observed even when treatment was initiated after surgical removal of the primary tumour. Taken together, these results indicate that [10]-gingerol may be a safe and useful complementary therapy for the treatment of metastatic breast cancer and warrant further investigation of its efficacy, either alone or in combination with standard systemic therapies, in pre-clinical models of metastatic breast cancer and in patients.
Keywords: animal models; apoptosis; breast cancer; cell cycle; gingerol.
Conflict of interest statement
CONFLICTS OF INTEREST Authors declare no conflicts of interest.
Figures



Similar articles
-
[10]-Gingerol Affects Multiple Metastatic Processes and Induces Apoptosis in MDAMB- 231 Breast Tumor Cells.Anticancer Agents Med Chem. 2019;19(5):645-654. doi: 10.2174/1871520618666181029125607. Anticancer Agents Med Chem. 2019. PMID: 30370858
-
[10]-Gingerol improves doxorubicin anticancer activity and decreases its side effects in triple negative breast cancer models.Cell Oncol (Dordr). 2020 Oct;43(5):915-929. doi: 10.1007/s13402-020-00539-z. Epub 2020 Aug 6. Cell Oncol (Dordr). 2020. PMID: 32761561
-
[6]-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer cells.J Nutr Biochem. 2008 May;19(5):313-9. doi: 10.1016/j.jnutbio.2007.05.008. Epub 2007 Aug 1. J Nutr Biochem. 2008. PMID: 17683926
-
[6]-Gingerol-Derived Semi-Synthetic Compound SSi6 Inhibits Tumor Growth and Metastatic Dissemination in Triple-Negative Breast Cancer Xenograft Models.Cancers (Basel). 2021 Jun 8;13(12):2855. doi: 10.3390/cancers13122855. Cancers (Basel). 2021. PMID: 34201040 Free PMC article.
-
Gingerol, a Natural Antioxidant, Attenuates Hyperglycemia and Downstream Complications.Metabolites. 2022 Dec 16;12(12):1274. doi: 10.3390/metabo12121274. Metabolites. 2022. PMID: 36557312 Free PMC article. Review.
Cited by
-
Identification of brain metastasis genes and therapeutic evaluation of histone deacetylase inhibitors in a clinically relevant model of breast cancer brain metastasis.Dis Model Mech. 2018 Jul 6;11(7):DMM034850. doi: 10.1242/dmm.034850. Dis Model Mech. 2018. PMID: 29784888 Free PMC article.
-
Insight into the Biological Roles and Mechanisms of Phytochemicals in Different Types of Cancer: Targeting Cancer Therapeutics.Nutrients. 2023 Mar 31;15(7):1704. doi: 10.3390/nu15071704. Nutrients. 2023. PMID: 37049544 Free PMC article. Review.
-
Research Trend and Detailed Insights into the Molecular Mechanisms of Food Bioactive Compounds against Cancer: A Comprehensive Review with Special Emphasis on Probiotics.Cancers (Basel). 2022 Nov 8;14(22):5482. doi: 10.3390/cancers14225482. Cancers (Basel). 2022. PMID: 36428575 Free PMC article. Review.
-
Gingers and Their Purified Components as Cancer Chemopreventative Agents.Molecules. 2019 Aug 7;24(16):2859. doi: 10.3390/molecules24162859. Molecules. 2019. PMID: 31394732 Free PMC article. Review.
-
Bifunctional Aptamer-Doxorubicin Conjugate Crosses the Blood-Brain Barrier and Selectively Delivers Its Payload to EpCAM-Positive Tumor Cells.Nucleic Acid Ther. 2020 Apr;30(2):117-128. doi: 10.1089/nat.2019.0807. Epub 2020 Feb 6. Nucleic Acid Ther. 2020. PMID: 32027209 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

