Corneal Tissue From Dry Eye Donors Leads to Enhanced Graft Rejection
- PMID: 29023237
- PMCID: PMC5716893
- DOI: 10.1097/ICO.0000000000001400
Corneal Tissue From Dry Eye Donors Leads to Enhanced Graft Rejection
Abstract
Purpose: To assess the effect of dry eye disease (DED) in graft donors on dendritic cell (DC) maturation, host T-cell sensitization, and corneal allograft rejection.
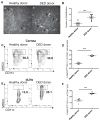
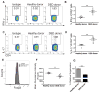
Methods: Corneas of control (healthy donor) and DED mice (C57BL/6) were transplanted onto fully allogeneic naive BALB/c recipients (n = 10 mice/group). Long-term allograft survival was evaluated for 8 weeks. Corneas and draining lymph nodes (dLNs) were harvested at posttransplantation day 14 (n = 5 mice/group). The frequencies of MHCII CD11c DCs in the donor corneas and host dLNs and the frequencies of interferon (IFN)-γ and IL-17 CD4 T cells and Foxp3 expression by Tregs in host dLNs were investigated using flow cytometry. The enzyme-linked immunospot assay was used to assess host T-cell allosensitization through direct and indirect pathways (n = 3/group).
Results: Recipients of DED donor corneas showed significantly reduced graft survival (10%) compared with control mice (50% survival, P = 0.022), and had significantly increased frequencies of mature DCs in the grafted cornea (DED donor 44.0% ± 0.36% vs. healthy donor 35.4 ± 0.5%; P < 0.0001) and host dLNs (DED donor 25.1% ± 0.66% vs. healthy donor 13.7% ± 1.6%; P = 0.005). Frequencies of IFN-γ and IL-17 T cells were increased in the dLNs of recipients of DED corneas, whereas the expression (mean fluorescence intensity) of Foxp3 in Tregs was decreased significantly in these mice (DED donor 6004 ± 193 vs. healthy donor 6806 ± 81; P = 0.0002). Enzyme-linked immunospot analysis showed that the direct pathway of allosensitization was significantly amplified in recipients of grafts with DED (P = 0.0146).
Conclusions: Our results indicate that DED in the donor is a significant risk factor for subsequent corneal allograft rejection.
Figures
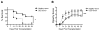

Similar articles
-
Endogenous Toll-like Receptor 2 Modulates Th1/Treg-Promoting Dendritic Cells in Mice Corneal Transplantation Model.Curr Eye Res. 2020 Jul;45(7):774-781. doi: 10.1080/02713683.2019.1705491. Epub 2019 Dec 26. Curr Eye Res. 2020. PMID: 31842628
-
Mesenchymal stem cells home to inflamed ocular surface and suppress allosensitization in corneal transplantation.Invest Ophthalmol Vis Sci. 2014 Sep 16;55(10):6631-8. doi: 10.1167/iovs.14-15413. Invest Ophthalmol Vis Sci. 2014. PMID: 25228546
-
Role of CCR7 in facilitating direct allosensitization and regulatory T-cell function in high-risk corneal transplantation.Invest Ophthalmol Vis Sci. 2010 Feb;51(2):816-21. doi: 10.1167/iovs.09-3952. Epub 2009 Sep 24. Invest Ophthalmol Vis Sci. 2010. PMID: 19797201 Free PMC article.
-
Role of resident corneal leukocytes and draining cervical lymph nodes in corneal allograft rejection.Cornea. 2003 Oct;22(7 Suppl):S61-5. doi: 10.1097/00003226-200310001-00009. Cornea. 2003. PMID: 14703709 Review.
-
Gene therapy approaches to prolonging corneal allograft survival.Expert Opin Biol Ther. 2004 Jul;4(7):1059-71. doi: 10.1517/14712598.4.7.1059. Expert Opin Biol Ther. 2004. PMID: 15268674 Review.
Cited by
-
Variable Responses to Corneal Grafts: Insights from Immunology and Systems Biology.J Clin Med. 2020 Feb 21;9(2):586. doi: 10.3390/jcm9020586. J Clin Med. 2020. PMID: 32098130 Free PMC article. Review.
-
Role of Immune Cell Diversity and Heterogeneity in Corneal Graft Survival: A Systematic Review and Meta-Analysis.J Clin Med. 2021 Oct 12;10(20):4667. doi: 10.3390/jcm10204667. J Clin Med. 2021. PMID: 34682792 Free PMC article. Review.
-
Regulatory T cell modulation of cytokine and cellular networks in corneal graft rejection.Curr Ophthalmol Rep. 2018 Dec;6(4):266-274. Epub 2018 Oct 6. Curr Ophthalmol Rep. 2018. PMID: 31807370 Free PMC article.
-
Ex Vivo-Induced Bone Marrow-Derived Myeloid Suppressor Cells Prevent Corneal Allograft Rejection in Mice.Invest Ophthalmol Vis Sci. 2021 Jun 1;62(7):3. doi: 10.1167/iovs.62.7.3. Invest Ophthalmol Vis Sci. 2021. PMID: 34061951 Free PMC article.
-
Dry Eye Disease: Emerging Approaches to Disease Analysis and Therapy.J Clin Med. 2019 Sep 11;8(9):1439. doi: 10.3390/jcm8091439. J Clin Med. 2019. PMID: 31514344 Free PMC article. Review.
References
-
- Williams KA, Brereton HM, Coster DJ. Prospects for genetic modulation of corneal graft survival. Eye. 2009;23:1904–9. - PubMed
-
- Dana MR, Qian Y, Hamrah P. Twenty-five-year panorama of corneal immunology: emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea. 2000;19:625–43. - PubMed
-
- Luo L, Li DQ, Doshi A, et al. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–301. - PubMed
-
- Stern ME, Beuerman RW, Fox RI, et al. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998;17:584–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

