Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057)
- PMID: 29023213
- PMCID: PMC6075826
- DOI: 10.1200/JCO.2017.74.3062
Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057)
Abstract
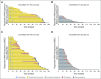
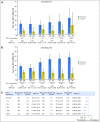
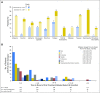
Purpose Nivolumab, a programmed death-1 inhibitor, prolonged overall survival compared with docetaxel in two independent phase III studies in previously treated patients with advanced squamous (CheckMate 017; ClinicalTrials.gov identifier: NCT01642004) or nonsquamous (CheckMate 057; ClinicalTrials.gov identifier: NCT01673867) non-small-cell lung cancer (NSCLC). We report updated results, including a pooled analysis of the two studies. Methods Patients with stage IIIB/IV squamous (N = 272) or nonsquamous (N = 582) NSCLC and disease progression during or after prior platinum-based chemotherapy were randomly assigned 1:1 to nivolumab (3 mg/kg every 2 weeks) or docetaxel (75 mg/m2 every 3 weeks). Minimum follow-up for survival was 24.2 months. Results Two-year overall survival rates with nivolumab versus docetaxel were 23% (95% CI, 16% to 30%) versus 8% (95% CI, 4% to 13%) in squamous NSCLC and 29% (95% CI, 24% to 34%) versus 16% (95% CI, 12% to 20%) in nonsquamous NSCLC; relative reductions in the risk of death with nivolumab versus docetaxel remained similar to those reported in the primary analyses. Durable responses were observed with nivolumab; 10 (37%) of 27 confirmed responders with squamous NSCLC and 19 (34%) of 56 with nonsquamous NSCLC had ongoing responses after 2 years' minimum follow-up. No patient in either docetaxel group had an ongoing response. In the pooled analysis, the relative reduction in the risk of death with nivolumab versus docetaxel was 28% (hazard ratio, 0.72; 95% CI, 0.62 to 0.84), and rates of treatment-related adverse events were lower with nivolumab than with docetaxel (any grade, 68% v 88%; grade 3 to 4, 10% v 55%). Conclusion Nivolumab provides long-term clinical benefit and a favorable tolerability profile compared with docetaxel in previously treated patients with advanced NSCLC.
Figures



Comment in
-
Nivolumab in pretreated non-small cell lung cancer: continuing the immunolution.Transl Lung Cancer Res. 2018 Apr;7(Suppl 2):S91-S94. doi: 10.21037/tlcr.2018.01.14. Transl Lung Cancer Res. 2018. PMID: 29782565 Free PMC article. No abstract available.
Similar articles
-
Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases.Ann Oncol. 2018 Apr 1;29(4):959-965. doi: 10.1093/annonc/mdy041. Ann Oncol. 2018. PMID: 29408986
-
Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer.N Engl J Med. 2015 Jul 9;373(2):123-35. doi: 10.1056/NEJMoa1504627. Epub 2015 May 31. N Engl J Med. 2015. PMID: 26028407 Free PMC article. Clinical Trial.
-
Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer.N Engl J Med. 2015 Oct 22;373(17):1627-39. doi: 10.1056/NEJMoa1507643. Epub 2015 Sep 27. N Engl J Med. 2015. PMID: 26412456 Free PMC article. Clinical Trial.
-
Nivolumab: A Review in Advanced Nonsquamous Non-Small Cell Lung Cancer.Drugs. 2016 Jun;76(9):969-78. doi: 10.1007/s40265-016-0589-9. Drugs. 2016. PMID: 27189706 Review.
-
Nivolumab: a review in advanced squamous non-small cell lung cancer.Drugs. 2015 Nov;75(16):1925-34. doi: 10.1007/s40265-015-0492-9. Drugs. 2015. PMID: 26514815 Review.
Cited by
-
Real-world treatment efficacy of anti-programmed death-1 combined with anti-angiogenesis therapy in non-small cell lung cancer patients.Medicine (Baltimore). 2020 Jun 12;99(24):e20545. doi: 10.1097/MD.0000000000020545. Medicine (Baltimore). 2020. PMID: 32541476 Free PMC article.
-
Establishment of a 5-gene risk model related to regulatory T cells for predicting gastric cancer prognosis.Cancer Cell Int. 2020 Sep 3;20:433. doi: 10.1186/s12935-020-01502-6. eCollection 2020. Cancer Cell Int. 2020. PMID: 32908454 Free PMC article.
-
Immune microenvironment composition in non-small cell lung cancer and its association with survival.Clin Transl Immunology. 2020 Jun 12;9(6):e1142. doi: 10.1002/cti2.1142. eCollection 2020. Clin Transl Immunology. 2020. PMID: 32547744 Free PMC article.
-
HLA Expression Correlates to the Risk of Immune Checkpoint Inhibitor-Induced Pneumonitis.Cells. 2020 Aug 25;9(9):1964. doi: 10.3390/cells9091964. Cells. 2020. PMID: 32854442 Free PMC article.
-
Longitudinal Evaluation of PD-L1 Expression on Circulating Tumor Cells in Non-Small Cell Lung Cancer Patients Treated with Nivolumab.Cancers (Basel). 2021 May 11;13(10):2290. doi: 10.3390/cancers13102290. Cancers (Basel). 2021. PMID: 34064720 Free PMC article.
References
-
- Torre LA, Bray F, Siegel RL, et al. : Global cancer statistics, 2012. CA Cancer J Clin 65:87-108, 2015 - PubMed
-
- https://www.cancer.org/cancer/non-small-cell-lung-cancer.html American Cancer Society: Non-small cell lung cancer.
-
- Melosky B, Chu Q, Juergens R, et al. : Pointed progress in second-line advanced non–small-cell lung cancer: The rapidly evolving field of checkpoint inhibition. J Clin Oncol 34:1676-1688, 2016 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

