Phase 1 trials of PEGylated recombinant human hyaluronidase PH20 in patients with advanced solid tumours
- PMID: 28949957
- PMCID: PMC5785735
- DOI: 10.1038/bjc.2017.327
Phase 1 trials of PEGylated recombinant human hyaluronidase PH20 in patients with advanced solid tumours
Erratum in
-
Phase 1 trials of PEGylated recombinant human hyaluronidase PH20 in patients with advanced solid tumours.Br J Cancer. 2018 Jan;118(2):e3. doi: 10.1038/bjc.2017.438. Br J Cancer. 2018. PMID: 29361630 Free PMC article.
Abstract
Background: Hyaluronan accumulation in tumour stroma is associated with reduced survival in preclinical cancer models. PEGPH20 degrades hyaluronan to facilitate tumour access for cancer therapies. Our objective was to assess safety and antitumour activity of PEGPH20 in patients with advanced solid tumours.
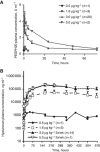
Methods: In HALO-109-101 (N=14), PEGPH20 was administered intravenously once or twice weekly (0.5 or 50 μg kg-1) or once every 3 weeks (0.5-1.5 μg kg-1). In HALO-109-102 (N=27), PEGPH20 was administered once or twice weekly (0.5-5.0 μg kg-1), with dexamethasone predose and postdose.
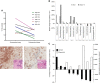
Results: Dose-limiting toxicities included grade ⩾3 myalgia, arthralgia, and muscle spasms; the maximum tolerated dose was 3.0 μg kg-1 twice weekly. Plasma hyaluronan increased in a dose-dependent manner, achieving steady state by Day 8 in multidose studies. A decrease in tumour hyaluronan level was observed in 5 of the 6 patients with pretreatment and posttreatment tumour biopsies. Exploratory imaging showed changes in tumour perfusion and decreased tumour metabolic activity, consistent with observations in animal models.
Conclusions: The tumour stroma has emerging importance in the development of cancer therapeutics. PEGPH20 3.0 μg kg-1 administered twice weekly is feasible in patients with advanced cancers; exploratory analyses indicate antitumour activity supporting further evaluation of PEGPH20 in solid tumours.
Conflict of interest statement
JRI reports that his institution has received research funding from Halozyme Therapeutics, Inc. RLK reports being a shareholder of Imaging Endpoints Core Lab. DDVH reports receiving a research grant to his institution from Halozyme Therapeutics, Inc. and is a consultant for Imaging Endpoints. LSR reports receiving a research grant to his institution from Halozyme Therapeutics, Inc. for the conduct of these clinical trials. PLR is currently on the DSMC for the pivotal phase 3 clinical trial of PEGPH20 in pancreatic cancer, sponsored by Halozyme Therapeutics, Inc., SSD is a shareholder of stock at Halozyme Therapeutics, Inc. and full-time employee at Fate Therapeutics. SSD, JZ, and GF were employees of Halozyme Therapeutics, Inc. at the time this trial was conducted. GF is a shareholder of stock at Halozyme Therapeutics, Inc. DCM, PJ, and HMS were employees of Halozyme Therapeutics, Inc. at the time this manuscript was submitted. RKR reports receiving research funding from Merrimack Pharmaceuticals. The other authors declare no conflict of interest.
Figures
Similar articles
-
Phase Ib Study of PEGylated Recombinant Human Hyaluronidase and Gemcitabine in Patients with Advanced Pancreatic Cancer.Clin Cancer Res. 2016 Jun 15;22(12):2848-54. doi: 10.1158/1078-0432.CCR-15-2010. Epub 2016 Jan 26. Clin Cancer Res. 2016. PMID: 26813359 Free PMC article. Clinical Trial.
-
Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models.Mol Cancer Ther. 2010 Nov;9(11):3052-64. doi: 10.1158/1535-7163.MCT-10-0470. Epub 2010 Oct 26. Mol Cancer Ther. 2010. PMID: 20978165
-
PEGylated recombinant human hyaluronidase (PEGPH20) pre-treatment improves intra-tumour distribution and efficacy of paclitaxel in preclinical models.J Exp Clin Cancer Res. 2021 Sep 10;40(1):286. doi: 10.1186/s13046-021-02070-x. J Exp Clin Cancer Res. 2021. PMID: 34507591 Free PMC article.
-
Targeting the Tumor Stroma: the Biology and Clinical Development of Pegylated Recombinant Human Hyaluronidase (PEGPH20).Curr Oncol Rep. 2017 Jul;19(7):47. doi: 10.1007/s11912-017-0608-3. Curr Oncol Rep. 2017. PMID: 28589527 Review.
-
ENHANZE® drug delivery technology: a novel approach to subcutaneous administration using recombinant human hyaluronidase PH20.Drug Deliv. 2019 Dec;26(1):98-106. doi: 10.1080/10717544.2018.1551442. Drug Deliv. 2019. PMID: 30744432 Free PMC article. Review.
Cited by
-
Characteristics of the Antitumor Effect of Doxorubicin and Pegylated Hyaluronidase on Models of Rat Brain Tumors.Bull Exp Biol Med. 2024 May;177(1):147-154. doi: 10.1007/s10517-024-06147-3. Epub 2024 Jul 4. Bull Exp Biol Med. 2024. PMID: 38963598
-
Multi-disciplinary Approach for Drug and Gene Delivery Systems to the Brain.AAPS PharmSciTech. 2021 Dec 3;23(1):11. doi: 10.1208/s12249-021-02144-1. AAPS PharmSciTech. 2021. PMID: 34862567 Free PMC article. Review.
-
Prolonged in vivo expression and anti-tumor response of DNA-based anti-HER2 antibodies.Oncotarget. 2018 Feb 6;9(17):13623-13636. doi: 10.18632/oncotarget.24426. eCollection 2018 Mar 2. Oncotarget. 2018. PMID: 29568382 Free PMC article.
-
Nanomedicine-based drug delivery towards tumor biological and immunological microenvironment.Acta Pharm Sin B. 2020 Nov;10(11):2110-2124. doi: 10.1016/j.apsb.2020.05.008. Epub 2020 May 31. Acta Pharm Sin B. 2020. PMID: 33304781 Free PMC article. Review.
-
Safety and pharmacokinetics of docetaxel in combination with pegvorhyaluronidase alfa in patients with non-small cell lung cancer.Clin Transl Sci. 2021 Sep;14(5):1875-1885. doi: 10.1111/cts.13041. Epub 2021 Jul 30. Clin Transl Sci. 2021. PMID: 33982408 Free PMC article. Clinical Trial.
References
-
- Abbott D, Comby P, Charuel C, Graepel P, Hanton G, Leblanc B, Lodola A, Longeart, Paulus L, Peters G, Stadler J (2004) Preclinical safety profile of sildenafil. Int J Impot Res 16: 498–504. - PubMed
-
- Abetamann V, Kern HF, Elsasser HP (1996) Differential expression of the hyaluronan receptors CD44 and RHAMM in human pancreatic cancer cells. Clin Cancer Res 2(9): 1607–1618. - PubMed
-
- Allen-Auerbach M, Weber WA (2009) Measuring response with FDG-PET: methodological aspects. Oncologist 14(4): 369–377. - PubMed
-
- Baumgartner G, Gomar-Hoss C, Sakr L, Ulsperger E, Wogritsch C (1998) The impact of extracellular matrix on the chemoresistance of solid tumors—experimental and clinical results of hyaluronidase as additive to cytostatic chemotherapy. Cancer Lett 131(1): 85–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

