Omega-3 PUFA ameliorates hyperhomocysteinemia-induced hepatic steatosis in mice by inhibiting hepatic ceramide synthesis
- PMID: 28933423
- PMCID: PMC5719150
- DOI: 10.1038/aps.2017.127
Omega-3 PUFA ameliorates hyperhomocysteinemia-induced hepatic steatosis in mice by inhibiting hepatic ceramide synthesis
Abstract
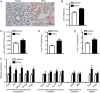
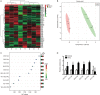
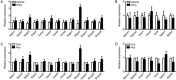
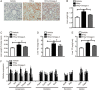
Hyperhomocysteinemia (HHcy) is a key risk factor in hepatic steatosis. In this study, we applied a metabolomic approach to investigate the changes in the metabolite profile due to HHcy-induced hepatic steatosis and the effects of omega-3 PUFA (polyunsaturated fatty acid) supplementation in mice. HHcy was induced in mice by giving DL-Hcy (1.8 g/L) in drinking water for 6 weeks, then the mice were sacrificed, and the metabolic profiles of the liver and plasma were analyzed through UPLC-ESI-QTOFMS-based lipidomics. Hepatic triglycerides and cholesterol were further assayed. The expression of ceramide metabolism-related genes was measured by quantitative PCR. Compared with control mice, HHcy mice exhibited hepatic steatosis with a notable increase in ceramide-related metabolites and subsequent upregulation of ceramide synthesis genes such as Sptlc3, Degs2, Cer4 and Smpd4. Omega-3 PUFA was simultaneously administered in HHcy mice through chow diet containing 3.3% omega-3 PUFA supplement for 6 weeks, which significantly ameliorated Hcy-induced hepatic steatosis. The decrease in hepatic lipid accumulation was mainly due to reduced hepatic levels of ceramides, which was partly the result of the lower expression of ceramide synthesis genes, Sptlc3 and Degs2. Similar beneficial effects of DHA were observed in Hcy-stimulated primary hepatocytes in vitro. In summary, Hcy-induced ceramide elevation in hepatocytes might contribute to the development of hepatic steatosis. Furthermore, downregulation of ceramide levels through omega-3 PUFA supplementation ameliorates hepatic lipid accumulation. Thus, ceramide is a potential therapeutic target for the treatment of hepatic steatosis.
Figures

Similar articles
-
[n-3 Polyunsaturated fatty acid attenuates hyperhomocysteinemia-induced hepatic steatosis by increasing hepatic LXA5 content].Sheng Li Xue Bao. 2021 Aug 25;73(4):551-558. Sheng Li Xue Bao. 2021. PMID: 34405211 Chinese.
-
Inhibition of soluble epoxide hydrolase ameliorates hyperhomocysteinemia-induced hepatic steatosis by enhancing β-oxidation of fatty acid in mice.Am J Physiol Gastrointest Liver Physiol. 2019 Apr 1;316(4):G527-G538. doi: 10.1152/ajpgi.00148.2018. Epub 2019 Feb 21. Am J Physiol Gastrointest Liver Physiol. 2019. PMID: 30789748 Free PMC article.
-
Hyperhomocysteinemia activates the aryl hydrocarbon receptor/CD36 pathway to promote hepatic steatosis in mice.Hepatology. 2016 Jul;64(1):92-105. doi: 10.1002/hep.28518. Epub 2016 Apr 5. Hepatology. 2016. PMID: 26928949
-
Orphan nuclear receptor NR4A1 suppresses hyperhomocysteinemia-induced hepatic steatosis in vitro and in vivo.FEBS Lett. 2019 May;593(10):1061-1071. doi: 10.1002/1873-3468.13384. Epub 2019 Apr 22. FEBS Lett. 2019. PMID: 30973961
-
Homocysteine Induces Hepatic Steatosis Involving ER Stress Response in High Methionine Diet-Fed Mice.Nutrients. 2017 Apr 1;9(4):346. doi: 10.3390/nu9040346. Nutrients. 2017. PMID: 28368295 Free PMC article.
Cited by
-
Insights into the roles and pathomechanisms of ceramide and sphigosine-1-phosphate in nonalcoholic fatty liver disease.Int J Biol Sci. 2023 Jan 1;19(1):311-330. doi: 10.7150/ijbs.78525. eCollection 2023. Int J Biol Sci. 2023. PMID: 36594091 Free PMC article. Review.
-
Lipids at the Nexus between Cerebrovascular Disease and Vascular Dementia: The Impact of HDL-Cholesterol and Ceramides.Int J Mol Sci. 2023 Feb 23;24(5):4403. doi: 10.3390/ijms24054403. Int J Mol Sci. 2023. PMID: 36901834 Free PMC article. Review.
-
The Lard Works in Mysterious Ways: Ceramides in Nutrition-Linked Chronic Disease.Annu Rev Nutr. 2022 Aug 22;42:115-144. doi: 10.1146/annurev-nutr-062220-112920. Epub 2022 May 18. Annu Rev Nutr. 2022. PMID: 35584813 Free PMC article. Review.
-
Deciphering the Link Between Hyperhomocysteinemia and Ceramide Metabolism in Alzheimer-Type Neurodegeneration.Front Neurol. 2019 Jul 31;10:807. doi: 10.3389/fneur.2019.00807. eCollection 2019. Front Neurol. 2019. PMID: 31417486 Free PMC article.
-
Effects of Fish Oil and Grape Seed Extract Combination on Hepatic Endogenous Antioxidants and Bioactive Lipids in Diet-Induced Early Stages of Insulin Resistance in Rats.Mar Drugs. 2020 Jun 16;18(6):318. doi: 10.3390/md18060318. Mar Drugs. 2020. PMID: 32560216 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

