Psoriasis pathogenesis and the development of novel targeted immune therapies
- PMID: 28887948
- PMCID: PMC5600287
- DOI: 10.1016/j.jaci.2017.07.004
Psoriasis pathogenesis and the development of novel targeted immune therapies
Abstract
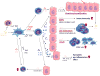
Psoriasis is caused by a complex interplay between the immune system, psoriasis-associated susceptibility loci, autoantigens, and multiple environmental factors. Over the last 2 decades, research has unequivocally shown that psoriasis represents a bona fide T cell-mediated disease primarily driven by pathogenic T cells that produce high levels of IL-17 in response to IL-23. The discovery of the central role for the IL-23/type 17 T-cell axis in the development of psoriasis has led to a major paradigm shift in the pathogenic model for this condition. The activation and upregulation of IL-17 in prepsoriatic skin produces a "feed forward" inflammatory response in keratinocytes that is self-amplifying and drives the development of mature psoriatic plaques by inducing epidermal hyperplasia, epidermal cell proliferation, and recruitment of leukocyte subsets into the skin. Clinical trial data for mAbs against IL-17 signaling (secukinumab, ixekizumab, and brodalumab) and newer IL-23p19 antagonists (tildrakizumab, guselkumab, and risankizumab) underscore the central role of these cytokines as predominant drivers of psoriatic disease. Currently, we are witnessing a translational revolution in the treatment and management of psoriasis. Emerging bispecific antibodies offer the potential for even better disease control, whereas small-molecule drugs offer future alternatives to the use of biologics and less costly long-term disease management.
Keywords: IL-17; IL-23; Psoriasis; autoantigens; brodalumab; ixekizumab; psoriatic arthritis; secukinumab; tildrakizumab, guselkumab, and risankizumab.
Copyright © 2017 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
JEH serves as an investigator for Pfizer Inc. JGK has been a consultant to and has received research support from companies that have developed or are developing therapeutics for psoriasis: AbbVie, Amgen, Boehringer, Bristol-Myers Squibb, Celgene, Dermira, Idera, Janssen, Leo, Lilly, Merck, Novartis, Pfizer, Regeneron, Sanofi, Serono, Sun, Valeant, and Vitae.
Figures

Similar articles
-
Discovery of the IL-23/IL-17 Signaling Pathway and the Treatment of Psoriasis.J Immunol. 2018 Sep 15;201(6):1605-1613. doi: 10.4049/jimmunol.1800013. J Immunol. 2018. PMID: 30181299 Free PMC article. Review.
-
Efficacy and safety of emerging immunotherapies in psoriasis.Immunotherapy. 2015;7(2):119-33. doi: 10.2217/imt.14.101. Immunotherapy. 2015. PMID: 25713988 Review.
-
Monoclonal antibodies inhibiting IL-12, -23, and -17 for the treatment of psoriasis.Hum Vaccin Immunother. 2017 Oct 3;13(10):2247-2259. doi: 10.1080/21645515.2017.1356498. Hum Vaccin Immunother. 2017. PMID: 28825875 Free PMC article. Review.
-
The role of IL 23 in the treatment of psoriasis.Expert Rev Clin Immunol. 2017 Jun;13(6):525-534. doi: 10.1080/1744666X.2017.1292137. Epub 2017 Feb 20. Expert Rev Clin Immunol. 2017. PMID: 28165883 Review.
-
New biologics in psoriasis: an update on IL-23 and IL-17 inhibitors.Cutis. 2017 Feb;99(2):123-127. Cutis. 2017. PMID: 28319618 Review.
Cited by
-
Review of Excessive Cytosolic DNA and Its Role in AIM2 and cGAS-STING Mediated Psoriasis Development.Clin Cosmet Investig Dermatol. 2024 Oct 22;17:2345-2357. doi: 10.2147/CCID.S476785. eCollection 2024. Clin Cosmet Investig Dermatol. 2024. PMID: 39464745 Free PMC article. Review.
-
Role of FOXM1 and AURKB in regulating keratinocyte function in psoriasis.Open Med (Wars). 2024 Sep 30;19(1):20241049. doi: 10.1515/med-2024-1049. eCollection 2024. Open Med (Wars). 2024. PMID: 39381423 Free PMC article.
-
Bioinformatic analysis of molecular characteristics and oncogenic features of CARD14 in human cancer.Sci Rep. 2024 Oct 3;14(1):22972. doi: 10.1038/s41598-024-74565-4. Sci Rep. 2024. PMID: 39362963 Free PMC article.
-
Cannabidiol Alleviates Imiquimod-Induced Psoriasis by Inhibiting JAK2-STAT3 in a Mouse Model.Biomedicines. 2024 Sep 12;12(9):2084. doi: 10.3390/biomedicines12092084. Biomedicines. 2024. PMID: 39335596 Free PMC article.
-
Ethosomes-mediated tryptanthrin delivery as efficient anti-psoriatic nanotherapy by enhancing topical drug absorption and lipid homeostasis.J Nanobiotechnology. 2024 Sep 28;22(1):584. doi: 10.1186/s12951-024-02860-3. J Nanobiotechnology. 2024. PMID: 39334378 Free PMC article.
References
-
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512–6. - PubMed
-
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509. - PubMed
-
- Gladman DD. Clinical Features and Diagnostic Considerations in Psoriatic Arthritis. Rheum Dis Clin North Am. 2015;41:569–79. - PubMed
-
- Brezinski EA, Dhillon JS, Armstrong AW. Economic Burden of Psoriasis in the United States: A Systematic Review. JAMA Dermatol. 2015;151:651–8. - PubMed
-
- Rapp SR, Feldman SR, Exum ML, Fleischer AB, Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41:401–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

