Microglia Morphological Categorization in a Rat Model of Neuroinflammation by Hierarchical Cluster and Principal Components Analysis
- PMID: 28848398
- PMCID: PMC5550745
- DOI: 10.3389/fncel.2017.00235
Microglia Morphological Categorization in a Rat Model of Neuroinflammation by Hierarchical Cluster and Principal Components Analysis
Abstract
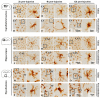
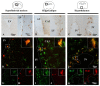
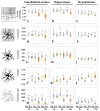
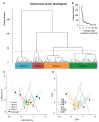
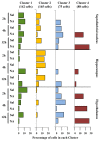
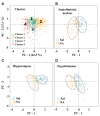
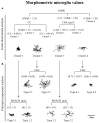
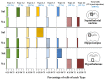
It is known that microglia morphology and function are closely related, but only few studies have objectively described different morphological subtypes. To address this issue, morphological parameters of microglial cells were analyzed in a rat model of aseptic neuroinflammation. After the injection of a single dose of the enzyme neuraminidase (NA) within the lateral ventricle (LV) an acute inflammatory process occurs. Sections from NA-injected animals and sham controls were immunolabeled with the microglial marker IBA1, which highlights ramifications and features of the cell shape. Using images obtained by section scanning, individual microglial cells were sampled from various regions (septofimbrial nucleus, hippocampus and hypothalamus) at different times post-injection (2, 4 and 12 h). Each cell yielded a set of 15 morphological parameters by means of image analysis software. Five initial parameters (including fractal measures) were statistically different in cells from NA-injected rats (most of them IL-1β positive, i.e., M1-state) compared to those from control animals (none of them IL-1β positive, i.e., surveillant state). However, additional multimodal parameters were revealed more suitable for hierarchical cluster analysis (HCA). This method pointed out the classification of microglia population in four clusters. Furthermore, a linear discriminant analysis (LDA) suggested three specific parameters to objectively classify any microglia by a decision tree. In addition, a principal components analysis (PCA) revealed two extra valuable variables that allowed to further classifying microglia in a total of eight sub-clusters or types. The spatio-temporal distribution of these different morphotypes in our rat inflammation model allowed to relate specific morphotypes with microglial activation status and brain location. An objective method for microglia classification based on morphological parameters is proposed. Main points Microglia undergo a quantifiable morphological change upon neuraminidase induced inflammation.Hierarchical cluster and principal components analysis allow morphological classification of microglia.Brain location of microglia is a relevant factor.
Keywords: fractal; microglia; morphofunctional; morphometric; neuraminidase.
Figures




Similar articles
-
Microglial Morphometric Parameters Correlate With the Expression Level of IL-1β, and Allow Identifying Different Activated Morphotypes.Front Cell Neurosci. 2019 Oct 25;13:472. doi: 10.3389/fncel.2019.00472. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31708746 Free PMC article.
-
Microglial activation by microbial neuraminidase through TLR2 and TLR4 receptors.J Neuroinflammation. 2019 Dec 2;16(1):245. doi: 10.1186/s12974-019-1643-9. J Neuroinflammation. 2019. PMID: 31791382 Free PMC article.
-
Microglial Metamorphosis in Three Dimensions in Virus Limbic Encephalitis: An Unbiased Pictorial Representation Based on a Stereological Sampling Approach of Surveillant and Reactive Microglia.Brain Sci. 2021 Jul 30;11(8):1009. doi: 10.3390/brainsci11081009. Brain Sci. 2021. PMID: 34439628 Free PMC article.
-
The Shape of μ-How Morphological Analyses Shape the Study of Microglia.Front Cell Neurosci. 2022 Jun 29;16:942462. doi: 10.3389/fncel.2022.942462. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35846562 Free PMC article. Review.
-
Microglial morphometric analysis: so many options, so little consistency.Front Neuroinform. 2023 Aug 10;17:1211188. doi: 10.3389/fninf.2023.1211188. eCollection 2023. Front Neuroinform. 2023. PMID: 37637472 Free PMC article. Review.
Cited by
-
A critical role for GM1 ganglioside in the pathophysiology and potential treatment of Parkinson's disease.Glycoconj J. 2022 Feb;39(1):13-26. doi: 10.1007/s10719-021-10002-2. Epub 2021 May 26. Glycoconj J. 2022. PMID: 34037912 Review.
-
Deepening the Mechanisms of Visceral Pain Persistence: An Evaluation of the Gut-Spinal Cord Relationship.Cells. 2020 Jul 24;9(8):1772. doi: 10.3390/cells9081772. Cells. 2020. PMID: 32722246 Free PMC article.
-
Differential regulation of microglial states by colony stimulating factors.Front Cell Neurosci. 2023 Oct 30;17:1275935. doi: 10.3389/fncel.2023.1275935. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37964794 Free PMC article. Review.
-
Modeling HIV-1 neuropathogenesis using three-dimensional human brain organoids (hBORGs) with HIV-1 infected microglia.Sci Rep. 2020 Sep 16;10(1):15209. doi: 10.1038/s41598-020-72214-0. Sci Rep. 2020. PMID: 32938988 Free PMC article.
-
Little cells of the little brain: microglia in cerebellar development and function.Trends Neurosci. 2021 Jul;44(7):564-578. doi: 10.1016/j.tins.2021.04.001. Epub 2021 Apr 28. Trends Neurosci. 2021. PMID: 33933255 Free PMC article. Review.
References
-
- Bernhardi R., Nicholls J. (1999). Transformation of leech microglial cell morphology and properties following co-culture with injured central nervous system tissue. J. Exp. Biol. 202, 723–728. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

