Low CD21 expression defines a population of recent germinal center graduates primed for plasma cell differentiation
- PMID: 28783670
- PMCID: PMC5896567
- DOI: 10.1126/sciimmunol.aai8153
Low CD21 expression defines a population of recent germinal center graduates primed for plasma cell differentiation
Abstract
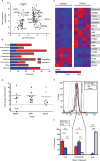
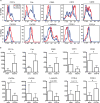
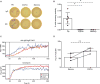
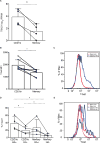
In this study, we report that antigen-specific CD19+CD27+CD21lo (CD21lo) B cells are transiently induced 14 to 28 days after immunization, at the time germinal centers (GCs) peak. Although clonally related to memory B cells and plasmablasts, CD21lo cells form distinct clades within phylogenetic trees based on accumulated variable gene mutations, supporting exit from active GCs. CD21lo cells express a transcriptional program, suggesting that they are primed for plasma cell differentiation and are refractory to GC differentiation, although they do not spontaneously secrete antibody. In addition, CD21lo cells differentially express multiple cell surface markers and have elevated intracellular levels of Blimp-1 and T-bet protein compared with memory B cells. Together, these data support a model in which CD21lo cells are recent GC graduates that represent a distinct population from CD27+ classical memory cells, are refractory to GC reentry, and are predisposed to differentiate into long-lived plasma cells.
Copyright © 2017, American Association for the Advancement of Science.
Conflict of interest statement
Figures


Similar articles
-
Complement receptors regulate differentiation of bone marrow plasma cell precursors expressing transcription factors Blimp-1 and XBP-1.J Exp Med. 2005 Mar 21;201(6):993-1005. doi: 10.1084/jem.20042239. Epub 2005 Mar 14. J Exp Med. 2005. PMID: 15767369 Free PMC article.
-
CD19loCD27hi Plasmablasts Suppress Harmful Th17 Inflammation Through Interleukin 10 Pathway in Colorectal Cancer.DNA Cell Biol. 2017 Oct;36(10):870-877. doi: 10.1089/dna.2017.3814. Epub 2017 Aug 22. DNA Cell Biol. 2017. PMID: 28829194
-
Human T-bet governs the generation of a distinct subset of CD11chighCD21low B cells.Sci Immunol. 2022 Jul 22;7(73):eabq3277. doi: 10.1126/sciimmunol.abq3277. Epub 2022 Jul 22. Sci Immunol. 2022. PMID: 35867801 Free PMC article.
-
Plasma cell and memory B cell differentiation from the germinal center.Curr Opin Immunol. 2017 Apr;45:97-102. doi: 10.1016/j.coi.2017.03.006. Epub 2017 Mar 17. Curr Opin Immunol. 2017. PMID: 28319733 Review.
-
Generation of memory B cells inside and outside germinal centers.Eur J Immunol. 2014 May;44(5):1258-64. doi: 10.1002/eji.201343716. Epub 2014 Apr 10. Eur J Immunol. 2014. PMID: 24610726 Review.
Cited by
-
Full-length single-cell BCR sequencing paired with RNA sequencing reveals convergent responses to pneumococcal vaccination.Commun Biol. 2024 Sep 28;7(1):1208. doi: 10.1038/s42003-024-06823-0. Commun Biol. 2024. PMID: 39341987 Free PMC article.
-
Modeling memory B cell responses in a lymphoid organ-chip to evaluate mRNA vaccine boosting.J Exp Med. 2024 Oct 7;221(10):e20240289. doi: 10.1084/jem.20240289. Epub 2024 Sep 6. J Exp Med. 2024. PMID: 39240335
-
Atypical and non-classical CD45RBlo memory B cells are the majority of circulating SARS-CoV-2 specific B cells following mRNA vaccination or COVID-19.Nat Commun. 2024 Aug 9;15(1):6811. doi: 10.1038/s41467-024-50997-4. Nat Commun. 2024. PMID: 39122676 Free PMC article.
-
Integrated analysis of tertiary lymphoid structures and immune infiltration in ccRCC microenvironment revealed their clinical significances: a multicenter cohort study.J Immunother Cancer. 2024 Jun 21;12(6):e008613. doi: 10.1136/jitc-2023-008613. J Immunother Cancer. 2024. PMID: 38908856 Free PMC article.
-
Children with perinatally acquired HIV exhibit distinct immune responses to 4CMenB vaccine.JCI Insight. 2024 Apr 11;9(10):e177182. doi: 10.1172/jci.insight.177182. JCI Insight. 2024. PMID: 38775152 Free PMC article.
References
-
- Victora GD, Nussenzweig MC. Germinal Centers. Annu Rev Immunol. 2012;30:429–457. - PubMed
-
- Dogan I, et al. Multiple layers of B cell memory with different effector functions. Nat Immunol. 2009;10:1292–1299. - PubMed
-
- McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-Specific Memory B Cell Development. Annu Rev Immunol. 2005;23:487–513. - PubMed
-
- Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral Immunity Due to Long-Lived Plasma Cells. Immunity. 1998;8:363–372. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

