Tumor Necrosis Factor dynamically regulates the mRNA stabilome in rheumatoid arthritis fibroblast-like synoviocytes
- PMID: 28708839
- PMCID: PMC5510804
- DOI: 10.1371/journal.pone.0179762
Tumor Necrosis Factor dynamically regulates the mRNA stabilome in rheumatoid arthritis fibroblast-like synoviocytes
Abstract
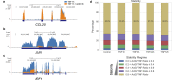
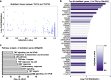
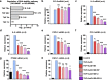
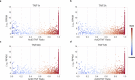
During rheumatoid arthritis (RA), Tumor Necrosis Factor (TNF) activates fibroblast-like synoviocytes (FLS) inducing in a temporal order a constellation of genes, which perpetuate synovial inflammation. Although the molecular mechanisms regulating TNF-induced transcription are well characterized, little is known about the impact of mRNA stability on gene expression and the impact of TNF on decay rates of mRNA transcripts in FLS. To address these issues we performed RNA sequencing and genome-wide analysis of the mRNA stabilome in RA FLS. We found that TNF induces a biphasic gene expression program: initially, the inducible transcriptome consists primarily of unstable transcripts but progressively switches and becomes dominated by very stable transcripts. This temporal switch is due to: a) TNF-induced prolonged stabilization of previously unstable transcripts that enables progressive transcript accumulation over days and b) sustained expression and late induction of very stable transcripts. TNF-induced mRNA stabilization in RA FLS occurs during the late phase of TNF response, is MAPK-dependent, and involves several genes with pathogenic potential such as IL6, CXCL1, CXCL3, CXCL8/IL8, CCL2, and PTGS2. These results provide the first insights into genome-wide regulation of mRNA stability in RA FLS and highlight the potential contribution of dynamic regulation of the mRNA stabilome by TNF to chronic synovitis.
Conflict of interest statement
Figures


Similar articles
-
Control of cytokine mRNA degradation by the histone deacetylase inhibitor ITF2357 in rheumatoid arthritis fibroblast-like synoviocytes: beyond transcriptional regulation.Arthritis Res Ther. 2018 Jul 20;20(1):148. doi: 10.1186/s13075-018-1638-4. Arthritis Res Ther. 2018. PMID: 30029685 Free PMC article.
-
Increased sensitivity of rheumatoid synoviocytes to Schnurri-3 expression in TNF-α and IL-17A induced osteoblastic differentiation.Bone. 2016 Jun;87:89-96. doi: 10.1016/j.bone.2016.04.008. Epub 2016 Apr 9. Bone. 2016. PMID: 27072520
-
Soluble CD14 Induces Pro-inflammatory Cytokines in Rheumatoid Arthritis Fibroblast-Like Synovial Cells via Toll-Like Receptor 4.Cells. 2020 Jul 14;9(7):1689. doi: 10.3390/cells9071689. Cells. 2020. PMID: 32674360 Free PMC article.
-
Stable activation of fibroblasts in rheumatic arthritis-causes and consequences.Rheumatology (Oxford). 2016 Dec;55(suppl 2):ii64-ii67. doi: 10.1093/rheumatology/kew347. Rheumatology (Oxford). 2016. PMID: 27856663 Review.
-
The Effects of MicroRNAs on Key Signalling Pathways and Epigenetic Modification in Fibroblast-Like Synoviocytes of Rheumatoid Arthritis.Mediators Inflamm. 2018 May 10;2018:9013124. doi: 10.1155/2018/9013124. eCollection 2018. Mediators Inflamm. 2018. PMID: 29861659 Free PMC article. Review.
Cited by
-
[Rheumatoid arthritis].Z Rheumatol. 2018 Nov;77(9):769-775. doi: 10.1007/s00393-018-0500-z. Z Rheumatol. 2018. PMID: 29974224 Review. German.
-
Short-term transcriptomic response to plasma membrane injury.Sci Rep. 2021 Sep 27;11(1):19141. doi: 10.1038/s41598-021-98420-y. Sci Rep. 2021. PMID: 34580330 Free PMC article.
-
IL6/sIL6R regulates TNFα-inflammatory response in synovial fibroblasts through modulation of transcriptional and post-transcriptional mechanisms.BMC Mol Cell Biol. 2020 Oct 30;21(1):74. doi: 10.1186/s12860-020-00317-7. BMC Mol Cell Biol. 2020. PMID: 33126846 Free PMC article.
-
Control of cytokine mRNA degradation by the histone deacetylase inhibitor ITF2357 in rheumatoid arthritis fibroblast-like synoviocytes: beyond transcriptional regulation.Arthritis Res Ther. 2018 Jul 20;20(1):148. doi: 10.1186/s13075-018-1638-4. Arthritis Res Ther. 2018. PMID: 30029685 Free PMC article.
-
Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes.Nat Rev Rheumatol. 2020 Jun;16(6):316-333. doi: 10.1038/s41584-020-0413-5. Epub 2020 May 11. Nat Rev Rheumatol. 2020. PMID: 32393826 Free PMC article. Review.
References
-
- McInnes IB, Schett G (2011) The pathogenesis of rheumatoid arthritis. N Engl J Med 365: 2205–2219. doi: 10.1056/NEJMra1004965 - DOI - PubMed
-
- Noss EH, Brenner MB (2008) The role and therapeutic implications of fibroblast-like synoviocytes in inflammation and cartilage erosion in rheumatoid arthritis. Immunol Rev 223: 252–270. doi: 10.1111/j.1600-065X.2008.00648.x - DOI - PubMed
-
- Kalliolias GD, Ivashkiv LB (2016) TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol 12: 49–62. doi: 10.1038/nrrheum.2015.169 - DOI - PMC - PubMed
-
- Bottini N, Firestein GS (2013) Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol 9: 24–33. doi: 10.1038/nrrheum.2012.190 - DOI - PMC - PubMed
-
- Neumann E, Lefevre S, Zimmermann B, Gay S, Muller-Ladner U (2010) Rheumatoid arthritis progression mediated by activated synovial fibroblasts. Trends Mol Med 16: 458–468. doi: 10.1016/j.molmed.2010.07.004 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

