Functional expression of calcium-permeable canonical transient receptor potential 4-containing channels promotes migration of medulloblastoma cells
- PMID: 28627017
- PMCID: PMC5556167
- DOI: 10.1113/JP274659
Functional expression of calcium-permeable canonical transient receptor potential 4-containing channels promotes migration of medulloblastoma cells
Abstract
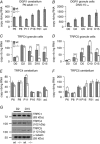
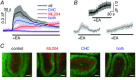
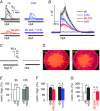
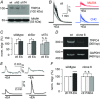
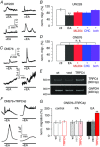
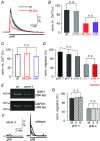
Key points: The proton sensing ovarian cancer G protein coupled receptor 1 (OGR1, aka GPR68) promotes expression of the canonical transient receptor potential channel subunit TRPC4 in normal and transformed cerebellar granule precursor (DAOY) cells. OGR1 and TRPC4 are prominently expressed in healthy cerebellar tissue throughout postnatal development and in primary cerebellar medulloblastoma tissues. Activation of TRPC4-containing channels in DAOY cells, but not non-transformed granule precursor cells, results in prominent increases in [Ca2+ ]i and promotes cell motility in wound healing and transwell migration assays. Medulloblastoma cells not arising from granule precursor cells show neither prominent rises in [Ca2+ ]i nor enhanced motility in response to TRPC4 activation unless they overexpressTRPC4. Our results suggest that OGR1 enhances expression of TRPC4-containing channels that contribute to enhanced invasion and metastasis of granule precursor-derived human medulloblastoma.
Abstract: Aberrant intracellular Ca2+ signalling contributes to the formation and progression of a range of distinct pathologies including cancers. Rises in intracellular Ca2+ concentration occur in response to Ca2+ influx through plasma membrane channels and Ca2+ release from intracellular Ca2+ stores, which can be mobilized in response to activation of cell surface receptors. Ovarian cancer G protein coupled receptor 1 (OGR1, aka GPR68) is a proton-sensing Gq -coupled receptor that is most highly expressed in cerebellum. Medulloblastoma (MB) is the most common paediatric brain tumour that arises from cerebellar precursor cells. We found that nine distinct human MB samples all expressed OGR1. In both normal granule cells and the transformed human cerebellar granule cell line DAOY, OGR1 promoted expression of the proton-potentiated member of the canonical transient receptor potential (TRPC) channel family, TRPC4. Consistent with a role for TRPC4 in MB, we found that all MB samples also expressed TRPC4. In DAOY cells, activation of TRPC4-containing channels resulted in large Ca2+ influx and enhanced migration, while in normal cerebellar granule (precursor) cells and MB cells not derived from granule precursors, only small levels of Ca2+ influx and no enhanced migration were observed. Our results suggest that OGR1-dependent increases in TRPC4 expression may favour formation of highly Ca2+ -permeable TRPC4-containing channels that promote transformed granule cell migration. Increased motility of cancer cells is a prerequisite for cancer invasion and metastasis, and our findings may point towards a key role for TRPC4 in progression of certain types of MB.
Keywords: OGR1; TRPC4; Transient Receptor Potential Channels; cerebellum; medulloblastoma; proton sensing G protein coupled receptors.
© 2017 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Figures

Similar articles
-
Functional expression of the proton sensors ASIC1a, TMEM206, and OGR1 together with BKCa channels is associated with cell volume changes and cell death under strongly acidic conditions in DAOY medulloblastoma cells.Pflugers Arch. 2024 Jun;476(6):923-937. doi: 10.1007/s00424-024-02964-7. Epub 2024 Apr 16. Pflugers Arch. 2024. PMID: 38627262 Free PMC article.
-
Activation of TRPC4β by Gαi subunit increases Ca2+ selectivity and controls neurite morphogenesis in cultured hippocampal neuron.Cell Calcium. 2013 Oct;54(4):307-19. doi: 10.1016/j.ceca.2013.07.006. Epub 2013 Aug 14. Cell Calcium. 2013. PMID: 24011658
-
Intracellular acidification facilitates receptor-operated TRPC4 activation through PLCδ1 in a Ca2+ -dependent manner.J Physiol. 2020 Jul;598(13):2651-2667. doi: 10.1113/JP279658. Epub 2020 May 22. J Physiol. 2020. PMID: 32338378 Free PMC article.
-
Role of Ca2+ signaling in the regulation of endothelial permeability.Vascul Pharmacol. 2002 Nov;39(4-5):173-85. doi: 10.1016/s1537-1891(03)00007-7. Vascul Pharmacol. 2002. PMID: 12747958 Review.
-
TRPC4- and TRPC4-containing channels.Handb Exp Pharmacol. 2014;222:85-128. doi: 10.1007/978-3-642-54215-2_5. Handb Exp Pharmacol. 2014. PMID: 24756704 Review.
Cited by
-
Calcium Signaling in Brain Cancers: Roles and Therapeutic Targeting.Cancers (Basel). 2019 Jan 26;11(2):145. doi: 10.3390/cancers11020145. Cancers (Basel). 2019. PMID: 30691160 Free PMC article. Review.
-
Proton-sensing ion channels, GPCRs and calcium signaling regulated by them: implications for cancer.Front Cell Dev Biol. 2024 Mar 5;12:1326231. doi: 10.3389/fcell.2024.1326231. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38505262 Free PMC article. Review.
-
Metabolite Sensing GPCRs: Promising Therapeutic Targets for Cancer Treatment?Cells. 2020 Oct 23;9(11):2345. doi: 10.3390/cells9112345. Cells. 2020. PMID: 33113952 Free PMC article. Review.
-
Mechanisms of Invasion in Glioblastoma: Extracellular Matrix, Ca2+ Signaling, and Glutamate.Front Cell Neurosci. 2021 Jun 2;15:663092. doi: 10.3389/fncel.2021.663092. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34149360 Free PMC article. Review.
-
Transient Receptor Potential Canonical (TRPC) Channels as Modulators of Migration and Invasion.Int J Mol Sci. 2020 Mar 3;21(5):1739. doi: 10.3390/ijms21051739. Int J Mol Sci. 2020. PMID: 32138386 Free PMC article. Review.
References
-
- Arcangeli A, Crociani O, Lastraioli E, Masi A, Pillozzi S & Becchetti A (2009). Targeting ion channels in cancer: a novel frontier in antineoplastic therapy. Curr Med Chem 16, 66–93. - PubMed
-
- Asghar MY, Magnusson M, Kemppainen K, Sukumaran P, Lof C, Pulli I, Kalhori V & Tornquist K (2015). Transient receptor potential canonical 1 (TRPC1) channels as regulators of sphingolipid and VEGF receptor expression: Implications for thyroid cancer cell migration and proliferation. J Biol Chem 290, 16116–16131. - PMC - PubMed
-
- Bartlett F, Kortmann R & Saran F (2013). Medulloblastoma. Clin Oncol 25, 36–45. - PubMed
-
- Becchetti A & Arcangeli A (2010). Integrins and ion channels in cell migration: implications for neuronal development, wound healing and metastatic spread. Adv Exp Med Biol 674, 107–123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

