Increased miltefosine tolerance in clinical isolates of Leishmania donovani is associated with reduced drug accumulation, increased infectivity and resistance to oxidative stress
- PMID: 28575060
- PMCID: PMC5470736
- DOI: 10.1371/journal.pntd.0005641
Increased miltefosine tolerance in clinical isolates of Leishmania donovani is associated with reduced drug accumulation, increased infectivity and resistance to oxidative stress
Abstract
Background: Miltefosine (MIL) is an oral antileishmanial drug used for treatment of visceral leishmaniasis (VL) in the Indian subcontinent. Recent reports indicate a significant decline in its efficacy with a high rate of relapse in VL as well as post kala-azar dermal leishmaniasis (PKDL). We investigated the parasitic factors apparently involved in miltefosine unresponsiveness in clinical isolates of Leishmania donovani.
Methodology: L. donovani isolated from patients of VL and PKDL at pretreatment stage (LdPreTx, n = 9), patients that relapsed after MIL treatment (LdRelapse, n = 7) and parasites made experimentally resistant to MIL (LdM30) were included in this study. MIL uptake was estimated using liquid chromatography coupled mass spectrometry. Reactive oxygen species and intracellular thiol content were measured fluorometrically. Q-PCR was used to assess the differential expression of genes associated with MIL resistance.
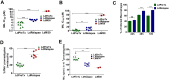
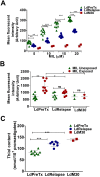
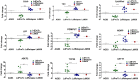
Results: LdRelapse parasites exhibited higher IC50 both at promastigote level (7.92 ± 1.30 μM) and at intracellular amastigote level (11.35 ± 6.48 μM) when compared with LdPreTx parasites (3.27 ± 1.52 μM) and (3.85 ± 3.11 μM), respectively. The percent infectivity (72 hrs post infection) of LdRelapse parasites was significantly higher (80.71 ± 5.67%, P<0.001) in comparison to LdPreTx (60.44 ± 2.80%). MIL accumulation was significantly lower in LdRelapse parasites (1.7 fold, P<0.001) and in LdM30 parasites (2.4 fold, P<0.001) when compared with LdPreTx parasites. MIL induced ROS levels were significantly lower (p<0.05) in macrophages infected with LdRelapse while intracellular thiol content were significantly higher in LdRelapse compared to LdPreTx, indicating a better tolerance for oxidative stress in LdRelapse isolates. Genes associated with oxidative stress, metabolic processes and transporters showed modulated expression in LdRelapse and LdM30 parasites in comparison with LdPreTx parasites.
Conclusion: The present study highlights the parasitic factors and pathways responsible for miltefosine unresponsiveness in VL and PKDL.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Drug susceptibility in Leishmania isolates following miltefosine treatment in cases of visceral leishmaniasis and post kala-azar dermal leishmaniasis.PLoS Negl Trop Dis. 2012;6(5):e1657. doi: 10.1371/journal.pntd.0001657. Epub 2012 May 22. PLoS Negl Trop Dis. 2012. PMID: 22629478 Free PMC article.
-
Antimony susceptible Leishmania donovani: evidence from in vitro drug susceptibility of parasites isolated from patients of post-kala-azar dermal leishmaniasis in pre- and post-miltefosine era.Microbiol Spectr. 2024 Jun 4;12(6):e0402623. doi: 10.1128/spectrum.04026-23. Epub 2024 May 7. Microbiol Spectr. 2024. PMID: 38712926 Free PMC article.
-
Lipase Precursor-Like Protein Promotes Miltefosine Tolerance in Leishmania donovani by Enhancing Parasite Infectivity and Eliciting Anti-inflammatory Responses in Host Macrophages.Antimicrob Agents Chemother. 2018 Nov 26;62(12):e00666-18. doi: 10.1128/AAC.00666-18. Print 2018 Dec. Antimicrob Agents Chemother. 2018. PMID: 30297367 Free PMC article.
-
Evaluating drug resistance in visceral leishmaniasis: the challenges.Parasitology. 2018 Apr;145(4):453-463. doi: 10.1017/S0031182016002031. Epub 2016 Nov 21. Parasitology. 2018. PMID: 27866478 Free PMC article. Review.
-
The preclinical discovery and development of oral miltefosine for the treatment of visceral leishmaniasis: a case history.Expert Opin Drug Discov. 2020 Jun;15(6):647-658. doi: 10.1080/17460441.2020.1743674. Epub 2020 Mar 23. Expert Opin Drug Discov. 2020. PMID: 32202449 Review.
Cited by
-
Preparation and characterization of artemether-loaded niosomes in Leishmania major-induced cutaneous leishmaniasis.Sci Rep. 2024 May 2;14(1):10073. doi: 10.1038/s41598-024-60883-0. Sci Rep. 2024. PMID: 38698123 Free PMC article.
-
ABC transporter inhibition by beauvericin partially overcomes drug resistance in Leishmania tropica.Antimicrob Agents Chemother. 2024 May 2;68(5):e0136823. doi: 10.1128/aac.01368-23. Epub 2024 Apr 4. Antimicrob Agents Chemother. 2024. PMID: 38572959 Free PMC article.
-
In Vitro and Ex Vivo Synergistic Effect of Pyrvinium Pamoate Combined with Miltefosine and Paromomycin against Leishmania.Trop Med Infect Dis. 2024 Jan 25;9(2):30. doi: 10.3390/tropicalmed9020030. Trop Med Infect Dis. 2024. PMID: 38393119 Free PMC article.
-
Systematic Review of Treatment Failure and Clinical Relapses in Leishmaniasis from a Multifactorial Perspective: Clinical Aspects, Factors Associated with the Parasite and Host.Trop Med Infect Dis. 2023 Aug 29;8(9):430. doi: 10.3390/tropicalmed8090430. Trop Med Infect Dis. 2023. PMID: 37755891 Free PMC article. Review.
-
Retrospective Long-Term Evaluation of Miltefosine-Allopurinol Treatment in Canine Leishmaniosis.Pathogens. 2023 Jun 22;12(7):864. doi: 10.3390/pathogens12070864. Pathogens. 2023. PMID: 37513711 Free PMC article.
References
-
- WHO (2010) Report of a meeting of the WHO expert committee on the control of Leishmaniases, Geneva, 22–26 March, 2010.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

