Diverse stimuli engage different neutrophil extracellular trap pathways
- PMID: 28574339
- PMCID: PMC5496738
- DOI: 10.7554/eLife.24437
Diverse stimuli engage different neutrophil extracellular trap pathways
Abstract
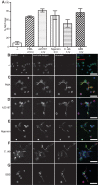
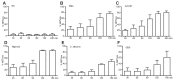
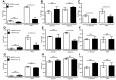
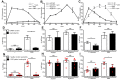
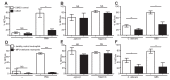
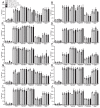
Neutrophils release neutrophil extracellular traps (NETs) which ensnare pathogens and have pathogenic functions in diverse diseases. We examined the NETosis pathways induced by five stimuli; PMA, the calcium ionophore A23187, nigericin, Candida albicans and Group B Streptococcus. We studied NET production in neutrophils from healthy donors with inhibitors of molecules crucial to PMA-induced NETs including protein kinase C, calcium, reactive oxygen species, the enzymes myeloperoxidase (MPO) and neutrophil elastase. Additionally, neutrophils from chronic granulomatous disease patients, carrying mutations in the NADPH oxidase complex or a MPO-deficient patient were examined. We show that PMA, C. albicans and GBS use a related pathway for NET induction, whereas ionophores require an alternative pathway but that NETs produced by all stimuli are proteolytically active, kill bacteria and composed mainly of chromosomal DNA. Thus, we demonstrate that NETosis occurs through several signalling mechanisms, suggesting that extrusion of NETs is important in host defence.
Keywords: NETs; Neutrophil; Neutrophil Extracellular Traps; cell biology; cell death; human; immunology; reactive oxygen species; signal transduction.
Conflict of interest statement
PRT: Consultant to Bristol-Myers Squibb.
The other authors declare that no competing interests exist.
Figures

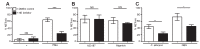

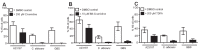
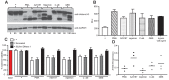
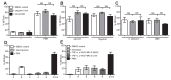
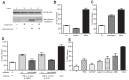
Similar articles
-
Mitochondrial permeability transition pore is involved in oxidative burst and NETosis of human neutrophils.Biochim Biophys Acta Mol Basis Dis. 2020 May 1;1866(5):165664. doi: 10.1016/j.bbadis.2020.165664. Epub 2020 Jan 8. Biochim Biophys Acta Mol Basis Dis. 2020. PMID: 31926265
-
Hypertonic Saline Suppresses NADPH Oxidase-Dependent Neutrophil Extracellular Trap Formation and Promotes Apoptosis.Front Immunol. 2018 Mar 8;9:359. doi: 10.3389/fimmu.2018.00359. eCollection 2018. Front Immunol. 2018. PMID: 29593709 Free PMC article.
-
A Label-Free Quantitative Proteomic Analysis of Mouse Neutrophil Extracellular Trap Formation Induced by Streptococcus suis or Phorbol Myristate Acetate (PMA).Front Immunol. 2018 Nov 13;9:2615. doi: 10.3389/fimmu.2018.02615. eCollection 2018. Front Immunol. 2018. PMID: 30555459 Free PMC article.
-
[Viewing sepsis and autoimmune disease in relation with infection and NETs-formation].Nihon Saikingaku Zasshi. 2018;73(2):171-191. doi: 10.3412/jsb.73.171. Nihon Saikingaku Zasshi. 2018. PMID: 29863035 Review. Japanese.
-
Neutrophil Extracellular Traps in Periodontitis: A Web of Intrigue.J Dent Res. 2016 Jan;95(1):26-34. doi: 10.1177/0022034515609097. Epub 2015 Oct 6. J Dent Res. 2016. PMID: 26442948 Review.
Cited by
-
Interplay between inflammation and thrombosis in cardiovascular pathology.Nat Rev Cardiol. 2021 Sep;18(9):666-682. doi: 10.1038/s41569-021-00552-1. Epub 2021 May 6. Nat Rev Cardiol. 2021. PMID: 33958774 Free PMC article. Review.
-
Anti-Apolipoprotein A-1 IgG Influences Neutrophil Extracellular Trap Content at Distinct Regions of Human Carotid Plaques.Int J Mol Sci. 2020 Oct 19;21(20):7721. doi: 10.3390/ijms21207721. Int J Mol Sci. 2020. PMID: 33086507 Free PMC article.
-
Neutrophils' Extracellular Trap Mechanisms: From Physiology to Pathology.Int J Mol Sci. 2022 Oct 25;23(21):12855. doi: 10.3390/ijms232112855. Int J Mol Sci. 2022. PMID: 36361646 Free PMC article. Review.
-
Cyclic stretch enhances neutrophil extracellular trap formation.BMC Biol. 2024 Sep 18;22(1):209. doi: 10.1186/s12915-024-02009-6. BMC Biol. 2024. PMID: 39289752 Free PMC article.
-
ATP amplifies NADPH-dependent and -independent neutrophil extracellular trap formation.Sci Rep. 2019 Nov 12;9(1):16556. doi: 10.1038/s41598-019-53058-9. Sci Rep. 2019. PMID: 31719610 Free PMC article.
References
-
- Barrientos L, Marin-Esteban V, de Chaisemartin L, Le-Moal VL, Sandré C, Bianchini E, Nicolas V, Pallardy M, Chollet-Martin S. An improved strategy to recover large fragments of functional human neutrophil extracellular traps. Frontiers in Immunology. 2013;4:166. doi: 10.3389/fimmu.2013.00166. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

