IL-2high tissue-resident T cells in the human liver: Sentinels for hepatotropic infection
- PMID: 28526759
- PMCID: PMC5461007
- DOI: 10.1084/jem.20162115
IL-2high tissue-resident T cells in the human liver: Sentinels for hepatotropic infection
Abstract
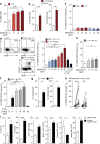
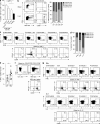
The liver provides a tolerogenic immune niche exploited by several highly prevalent pathogens as well as by primary and metastatic tumors. We have sampled healthy and hepatitis B virus (HBV)-infected human livers to probe for a subset of T cells specialized to overcome local constraints and mediate immunity. We characterize a population of T-betloEomesloBlimp-1hiHobitlo T cells found within the intrahepatic but not the circulating memory CD8 T cell pool expressing liver-homing/retention markers (CD69+CD103+ CXCR6+CXCR3+). These tissue-resident memory T cells (TRM) are preferentially expanded in patients with partial immune control of HBV infection and can remain in the liver after the resolution of infection, including compartmentalized responses against epitopes within all major HBV proteins. Sequential IL-15 or antigen exposure followed by TGFβ induces liver-adapted TRM, including their signature high expression of exhaustion markers PD-1 and CD39. We suggest that these inhibitory molecules, together with paradoxically robust, rapid, cell-autonomous IL-2 and IFNγ production, equip liver CD8 TRM to survive while exerting local noncytolytic hepatic immunosurveillance.
© 2017 Pallett et al.
Figures


Comment in
-
Tissue-resident T cells in hepatitis B: A new target for cure?J Exp Med. 2017 Jun 5;214(6):1564-1566. doi: 10.1084/jem.20170842. Epub 2017 May 19. J Exp Med. 2017. PMID: 28526760 Free PMC article.
Similar articles
-
Human intrahepatic CD69 + CD8+ T cells have a tissue resident memory T cell phenotype with reduced cytolytic capacity.Sci Rep. 2017 Jul 21;7(1):6172. doi: 10.1038/s41598-017-06352-3. Sci Rep. 2017. PMID: 28733665 Free PMC article.
-
Functional Heterogeneity of CD4+ Tumor-Infiltrating Lymphocytes With a Resident Memory Phenotype in NSCLC.Front Immunol. 2018 Nov 16;9:2654. doi: 10.3389/fimmu.2018.02654. eCollection 2018. Front Immunol. 2018. PMID: 30505306 Free PMC article.
-
Fine needle aspirates comprehensively sample intrahepatic immunity.Gut. 2019 Aug;68(8):1493-1503. doi: 10.1136/gutjnl-2018-317071. Epub 2018 Nov 28. Gut. 2019. PMID: 30487267 Free PMC article.
-
Liver-Resident Memory CD8+ T Cells: Possible Roles in Chronic HBV Infection.Int J Mol Sci. 2020 Dec 30;22(1):283. doi: 10.3390/ijms22010283. Int J Mol Sci. 2020. PMID: 33396596 Free PMC article. Review.
-
T-cell exhaustion in chronic hepatitis B infection: current knowledge and clinical significance.Cell Death Dis. 2015 Mar 19;6(3):e1694. doi: 10.1038/cddis.2015.42. Cell Death Dis. 2015. PMID: 25789969 Free PMC article. Review.
Cited by
-
The emerging role of effector functions exerted by tissue-resident memory T cells.Oxf Open Immunol. 2024 Jun 14;5(1):iqae006. doi: 10.1093/oxfimm/iqae006. eCollection 2024. Oxf Open Immunol. 2024. PMID: 39193473 Free PMC article. Review.
-
Role of the type 3 cytokines IL-17 and IL-22 in modulating metabolic dysfunction-associated steatotic liver disease.Front Immunol. 2024 Aug 2;15:1437046. doi: 10.3389/fimmu.2024.1437046. eCollection 2024. Front Immunol. 2024. PMID: 39156888 Free PMC article. Review.
-
Single cell RNA-sequencing identifies the effect of Normothermic ex vivo liver perfusion on liver-resident T cells.Transpl Immunol. 2024 Oct;86:102104. doi: 10.1016/j.trim.2024.102104. Epub 2024 Aug 13. Transpl Immunol. 2024. PMID: 39128812
-
TCF1-positive and TCF1-negative TRM CD8 T cell subsets and cDC1s orchestrate melanoma protection and immunotherapy response.J Immunother Cancer. 2024 Jul 5;12(7):e008739. doi: 10.1136/jitc-2023-008739. J Immunother Cancer. 2024. PMID: 38969523 Free PMC article.
-
Tissue-resident memory T cells break tolerance to renal autoantigens and orchestrate immune-mediated nephritis.Cell Mol Immunol. 2024 Sep;21(9):1066-1081. doi: 10.1038/s41423-024-01197-z. Epub 2024 Jul 3. Cell Mol Immunol. 2024. PMID: 38961265 Free PMC article.
References
-
- Boni C., Laccabue D., Lampertico P., Giuberti T., Viganò M., Schivazappa S., Alfieri A., Pesci M., Gaeta G.B., Brancaccio G., et al. . 2012. Restored function of HBV-specific T cells after long-term effective therapy with nucleos(t)ide analogues. Gastroenterology. 143:963–73.e9. 10.1053/j.gastro.2012.07.014 - DOI - PubMed
-
- Cheuk S., Schlums H., Gallais Sérézal I., Martini E., Chiang S.C., Marquardt N., Gibbs A., Detlofsson E., Introini A., Forkel M., et al. . 2017. CD49a expression defines tissue-resident CD8+ T cells poised for cytotoxic function in human skin. Immunity. 46:287–300. 10.1016/j.immuni.2017.01.009 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

