Rhinovirus C targets ciliated airway epithelial cells
- PMID: 28472984
- PMCID: PMC5418766
- DOI: 10.1186/s12931-017-0567-0
Rhinovirus C targets ciliated airway epithelial cells
Abstract
Background: The Rhinovirus C (RV-C), first identified in 2006, produce high symptom burdens in children and asthmatics, however, their primary target host cell in the airways remains unknown. Our primary hypotheses were that RV-C target ciliated airway epithelial cells (AECs), and that cell specificity is determined by restricted and high expression of the only known RV-C cell-entry factor, cadherin related family member 3 (CDHR3).
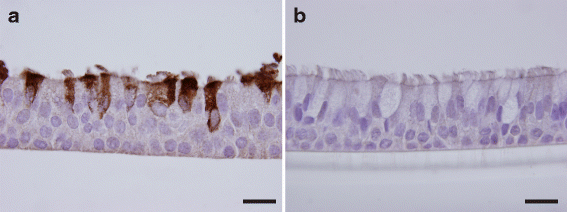
Methods: RV-C15 (C15) infection in differentiated human bronchial epithelial cell (HBEC) cultures was assessed using immunofluorescent and time-lapse epifluorescent imaging. Morphology of C15-infected differentiated AECs was assessed by immunohistochemistry.
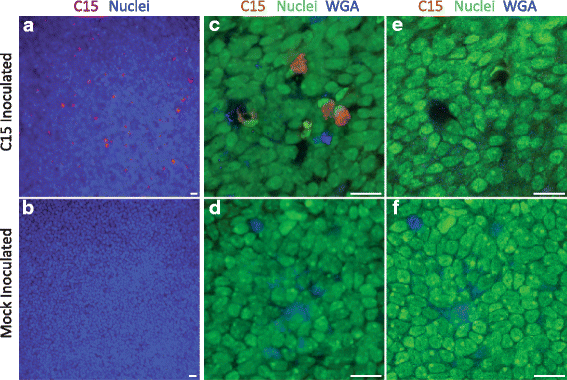
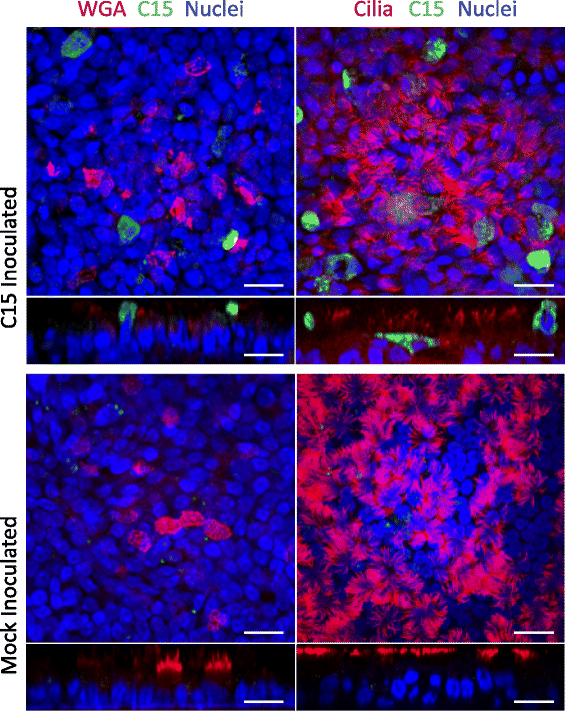
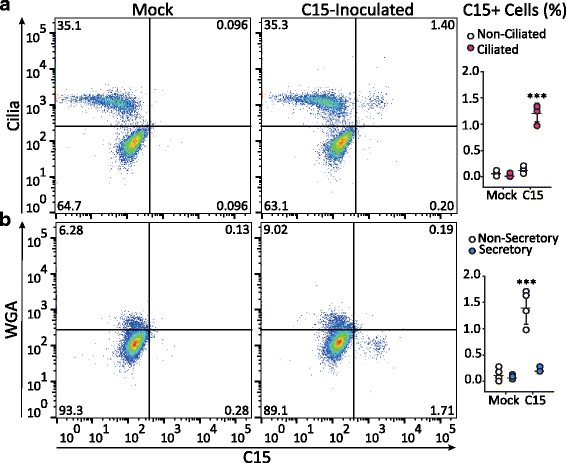
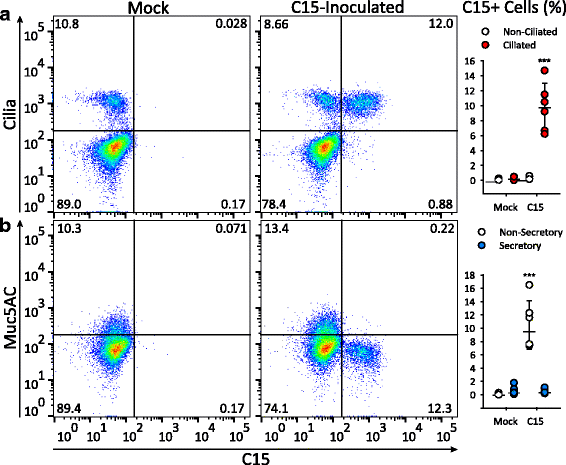
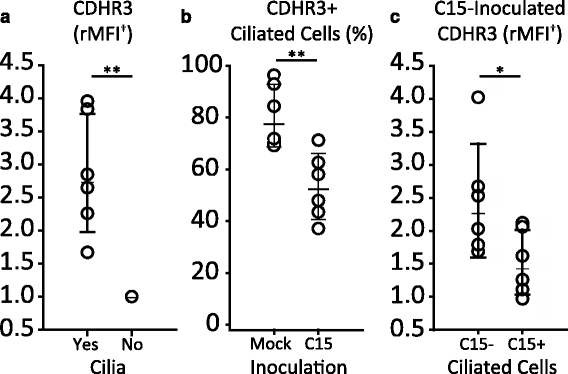
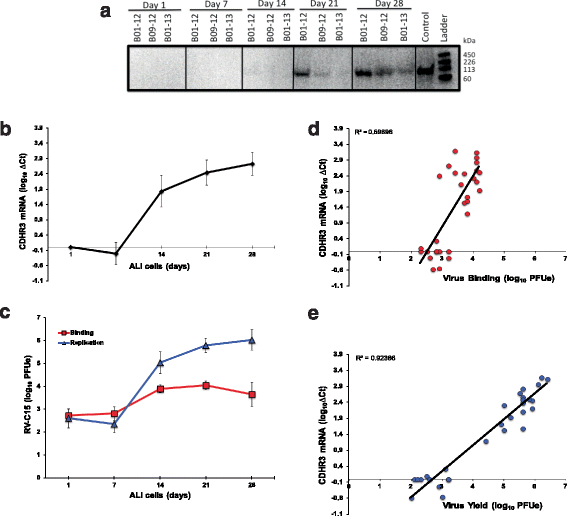
Results: C15 produced a scattered pattern of infection, and infected cells were shed from the epithelium. The percentage of cells infected with C15 varied from 1.4 to 14.7% depending on cell culture conditions. Infected cells had increased staining for markers of ciliated cells (acetylated-alpha-tubulin [aat], p < 0.001) but not markers of goblet cells (wheat germ agglutinin or Muc5AC, p = ns). CDHR3 expression was increased on ciliated epithelial cells, but not other epithelial cells (p < 0.01). C15 infection caused a 27.4% reduction of ciliated cells expressing CDHR3 (p < 0.01). During differentiation of AECs, CDHR3 expression progressively increased and correlated with both RV-C binding and replication.
Conclusions: The RV-C only replicate in ciliated AECs in vitro, leading to infected cell shedding. CDHR3 expression positively correlates with RV-C binding and replication, and is largely confined to ciliated AECs. Our data imply that factors regulating differentiation and CDHR3 production may be important determinants of RV-C illness severity.
Keywords: Air-liquid interface; Bronchial epithelium; CDHR3; Ciliated cells; Rhinovirus C.
Figures
Similar articles
-
CDHR3 Asthma-Risk Genotype Affects Susceptibility of Airway Epithelium to Rhinovirus C Infections.Am J Respir Cell Mol Biol. 2019 Oct;61(4):450-458. doi: 10.1165/rcmb.2018-0220OC. Am J Respir Cell Mol Biol. 2019. PMID: 30916989 Free PMC article.
-
Rhinovirus C replication is associated with the endoplasmic reticulum and triggers cytopathic effects in an in vitro model of human airway epithelium.PLoS Pathog. 2022 Jan 7;18(1):e1010159. doi: 10.1371/journal.ppat.1010159. eCollection 2022 Jan. PLoS Pathog. 2022. PMID: 34995322 Free PMC article.
-
Rhinovirus C Infection Induces Type 2 Innate Lymphoid Cell Expansion and Eosinophilic Airway Inflammation.Front Immunol. 2021 Apr 22;12:649520. doi: 10.3389/fimmu.2021.649520. eCollection 2021. Front Immunol. 2021. PMID: 33968043 Free PMC article.
-
Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication.Proc Natl Acad Sci U S A. 2015 Apr 28;112(17):5485-90. doi: 10.1073/pnas.1421178112. Epub 2015 Apr 6. Proc Natl Acad Sci U S A. 2015. PMID: 25848009 Free PMC article.
-
SARS-CoV replication and pathogenesis in an in vitro model of the human conducting airway epithelium.Virus Res. 2008 Apr;133(1):33-44. doi: 10.1016/j.virusres.2007.03.013. Epub 2007 Apr 23. Virus Res. 2008. PMID: 17451829 Free PMC article. Review.
Cited by
-
PGC-1α mediates a metabolic host defense response in human airway epithelium during rhinovirus infections.Nat Commun. 2021 Jun 16;12(1):3669. doi: 10.1038/s41467-021-23925-z. Nat Commun. 2021. PMID: 34135327 Free PMC article.
-
Conditionally reprogrammed asthmatic bronchial epithelial cells express lower FOXJ1 at terminal differentiation and lower IFNs following RV-A1 infection.Am J Physiol Lung Cell Mol Physiol. 2022 Oct 1;323(4):L495-L502. doi: 10.1152/ajplung.00230.2022. Epub 2022 Aug 30. Am J Physiol Lung Cell Mol Physiol. 2022. PMID: 36041223 Free PMC article.
-
Single-cell longitudinal analysis of SARS-CoV-2 infection in human airway epithelium identifies target cells, alterations in gene expression, and cell state changes.PLoS Biol. 2021 Mar 17;19(3):e3001143. doi: 10.1371/journal.pbio.3001143. eCollection 2021 Mar. PLoS Biol. 2021. PMID: 33730024 Free PMC article.
-
First contact: the role of respiratory cilia in host-pathogen interactions in the airways.Am J Physiol Lung Cell Mol Physiol. 2020 Oct 1;319(4):L603-L619. doi: 10.1152/ajplung.00283.2020. Epub 2020 Aug 12. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 32783615 Free PMC article. Review.
-
Elucidating the Interaction of CF Airway Epithelial Cells and Rhinovirus: Using the Host-Pathogen Relationship to Identify Future Therapeutic Strategies.Front Pharmacol. 2018 Nov 7;9:1270. doi: 10.3389/fphar.2018.01270. eCollection 2018. Front Pharmacol. 2018. PMID: 30464745 Free PMC article. Review.
References
-
- Jakab GJ. Mechanisms of bacterial superinfections in viral pneumonias. Schweiz Med Wochenschr. 1985;115(3):75–86. - PubMed
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

