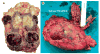
Renal cell carcinoma
- PMID: 28276433
- PMCID: PMC5936048
- DOI: 10.1038/nrdp.2017.9
Renal cell carcinoma
Abstract
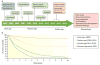
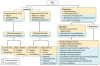
Renal cell carcinoma (RCC) denotes cancer originated from the renal epithelium and accounts for >90% of cancers in the kidney. The disease encompasses >10 histological and molecular subtypes, of which clear cell RCC (ccRCC) is most common and accounts for most cancer-related deaths. Although somatic VHL mutations have been described for some time, more-recent cancer genomic studies have identified mutations in epigenetic regulatory genes and demonstrated marked intra-tumour heterogeneity, which could have prognostic, predictive and therapeutic relevance. Localized RCC can be successfully managed with surgery, whereas metastatic RCC is refractory to conventional chemotherapy. However, over the past decade, marked advances in the treatment of metastatic RCC have been made, with targeted agents including sorafenib, sunitinib, bevacizumab, pazopanib and axitinib, which inhibit vascular endothelial growth factor (VEGF) and its receptor (VEGFR), and everolimus and temsirolimus, which inhibit mechanistic target of rapamycin complex 1 (mTORC1), being approved. Since 2015, agents with additional targets aside from VEGFR have been approved, such as cabozantinib and lenvatinib; immunotherapies, such as nivolumab, have also been added to the armamentarium for metastatic RCC. Here, we provide an overview of the biology of RCC, with a focus on ccRCC, as well as updates to complement the current clinical guidelines and an outline of potential future directions for RCC research and therapy.
Conflict of interest statement
J.J.H. is a consultant for Novartis, Eisai and Chugai and received research funding from Pfizer, Novartis, Eisai and Cancer Genomics Inc. C.S. is a consultant for Roche, Pfizer, Boehringer Ingelheim, Novartis, Celgene, Servler, Eli Lilly, and Glaxo Smithkline, and owns stock options from Achilles Therapeutics, Epic Biosciences, Grail, and Apogen Biotech. L.A. is a consultant for Pfizer, Novartis, Sanofi, Amgen, Bristol-Myers Squibb, Bayer and Cerulean, and received research funding from Pfizer and Novartis. M.S. is a consultant for Pfizer, Bristol-Myers Squibb, Ipsen, Exelixis, Eisai, Roche, Novartis and Astellas. D.Y.H. is a consultant for Pfizer, Novartis and Bristol-Myers Squibb. J.L. received research funding from Novartis, Pfizer, Bristol-Myers Squibb, and Merck Sharp & Dohme. M.P.P., S.S. and V.F. declare no competing interests.
Figures

Similar articles
-
Improvement in survival end points of patients with metastatic renal cell carcinoma through sequential targeted therapy.Cancer Treat Rev. 2016 Nov;50:109-117. doi: 10.1016/j.ctrv.2016.09.002. Epub 2016 Sep 10. Cancer Treat Rev. 2016. PMID: 27664394 Review.
-
A Systematic Review and Meta-analysis Comparing the Effectiveness and Adverse Effects of Different Systemic Treatments for Non-clear Cell Renal Cell Carcinoma.Eur Urol. 2017 Mar;71(3):426-436. doi: 10.1016/j.eururo.2016.11.020. Epub 2016 Dec 8. Eur Urol. 2017. PMID: 27939075 Review.
-
Systemic therapy in metastatic renal cell carcinoma.World J Urol. 2017 Feb;35(2):179-188. doi: 10.1007/s00345-016-1868-5. Epub 2016 Jun 9. World J Urol. 2017. PMID: 27277600 Free PMC article. Review.
-
Clinical management of metastatic kidney cancer: the role of new molecular drugs.Future Oncol. 2016 Jan;12(1):83-93. doi: 10.2217/fon.15.283. Epub 2015 Nov 30. Future Oncol. 2016. PMID: 26617188 Review.
-
Targeted Therapy for Metastatic Renal Cell Carcinoma.Acta Med Indones. 2016 Oct;48(4):335-347. Acta Med Indones. 2016. PMID: 28143997 Review.
Cited by
-
Identification of TYROBP and FCER1G as Key Genes with Prognostic Value in Clear Cell Renal Cell Carcinoma by Bioinformatics Analysis.Biochem Genet. 2021 Oct;59(5):1278-1294. doi: 10.1007/s10528-021-10061-y. Epub 2021 Mar 30. Biochem Genet. 2021. PMID: 33786672
-
System biology approaches identified novel biomarkers and their signaling pathways involved in renal cell carcinoma with different human diseases.Biosci Rep. 2022 Nov 30;42(11):BSR20221108. doi: 10.1042/BSR20221108. Biosci Rep. 2022. PMID: 36314741 Free PMC article.
-
Neuroendocrine gene subsets are uniquely dysregulated in prostate adenocarcinoma.Cancer Biol Ther. 2024 Dec 31;25(1):2364433. doi: 10.1080/15384047.2024.2364433. Epub 2024 Jun 26. Cancer Biol Ther. 2024. PMID: 38926911 Free PMC article.
-
Cuproptosis gene-related, neural network-based prognosis prediction and drug-target prediction for KIRC.Cancer Med. 2024 Jan;13(1):e6763. doi: 10.1002/cam4.6763. Epub 2023 Dec 22. Cancer Med. 2024. PMID: 38131663 Free PMC article.
-
Comprehensive Analysis of Transcriptional Expression of hsa-mir-21 Predicted Target Genes and Immune Characteristics in Kidney Renal Clear Cell Carcinoma.Int J Med Sci. 2022 Aug 21;19(9):1482-1501. doi: 10.7150/ijms.73404. eCollection 2022. Int J Med Sci. 2022. PMID: 36035369 Free PMC article.
References
-
- Eble JN, Sauter G, Epstein JI, Sesterhenn IA. World health organization classification of tumours. IARC Press; Lyon: 2004.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

