Mitochondria, Cybrids, Aging, and Alzheimer's Disease
- PMID: 28253988
- PMCID: PMC5864124
- DOI: 10.1016/bs.pmbts.2016.12.017
Mitochondria, Cybrids, Aging, and Alzheimer's Disease
Abstract
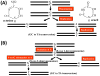
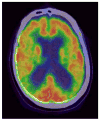
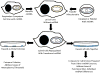
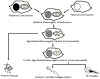
Mitochondrial and bioenergetic function change with advancing age and may drive aging phenotypes. Mitochondrial and bioenergetic changes are also documented in various age-related neurodegenerative diseases, including Alzheimer's disease (AD). In some instances AD mitochondrial and bioenergetic changes are reminiscent of those observed with advancing age but are greater in magnitude. Mitochondrial and bioenergetic dysfunction could, therefore, link neurodegeneration to brain aging. Interestingly, mitochondrial defects in AD patients are not brain-limited, and mitochondrial function can be linked to classic AD histologic changes including amyloid precursor protein processing to beta amyloid. Also, transferring mitochondria from AD subjects to cell lines depleted of endogenous mitochondrial DNA (mtDNA) creates cytoplasmic hybrid (cybrid) cell lines that recapitulate specific biochemical, molecular, and histologic AD features. Such findings have led to the formulation of a "mitochondrial cascade hypothesis" that places mitochondrial dysfunction at the apex of the AD pathology pyramid. Data pertinent to this premise are reviewed.
Keywords: Aging; Alzheimer's disease; Bioenergetics cybrids; Mitochondria.
© 2017 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Use of cytoplasmic hybrid cell lines for elucidating the role of mitochondrial dysfunction in Alzheimer's disease and Parkinson's disease.Ann N Y Acad Sci. 1999;893:176-91. doi: 10.1111/j.1749-6632.1999.tb07825.x. Ann N Y Acad Sci. 1999. PMID: 10672237 Review.
-
Bioenergetic flux, mitochondrial mass and mitochondrial morphology dynamics in AD and MCI cybrid cell lines.Hum Mol Genet. 2013 Oct 1;22(19):3931-46. doi: 10.1093/hmg/ddt247. Epub 2013 Jun 4. Hum Mol Genet. 2013. PMID: 23740939 Free PMC article.
-
Mitochondrial genomic contribution to mitochondrial dysfunction in Alzheimer's disease.J Alzheimers Dis. 2006 Jul;9(2):183-93. doi: 10.3233/jad-2006-9210. J Alzheimers Dis. 2006. PMID: 16873965 Review.
-
Abnormal mitochondrial morphology in sporadic Parkinson's and Alzheimer's disease cybrid cell lines.Exp Neurol. 2000 Mar;162(1):37-50. doi: 10.1006/exnr.2000.7333. Exp Neurol. 2000. PMID: 10716887
-
Mitochondria dysfunction of Alzheimer's disease cybrids enhances Abeta toxicity.J Neurochem. 2004 Jun;89(6):1417-26. doi: 10.1111/j.1471-4159.2004.02438.x. J Neurochem. 2004. PMID: 15189344
Cited by
-
The Rat Brain Transcriptome: From Infancy to Aging and Sporadic Alzheimer's Disease-like Pathology.Int J Mol Sci. 2023 Jan 11;24(2):1462. doi: 10.3390/ijms24021462. Int J Mol Sci. 2023. PMID: 36674977 Free PMC article.
-
Treatment of age-related visual impairment with a peptide acting on mitochondria.Dis Model Mech. 2022 Mar 1;15(3):dmm048256. doi: 10.1242/dmm.048256. Epub 2022 Feb 21. Dis Model Mech. 2022. PMID: 34766182 Free PMC article.
-
Horizons in Human Aging Neuroscience: From Normal Neural Aging to Mental (Fr)Agility.Front Hum Neurosci. 2022 Jun 29;16:815759. doi: 10.3389/fnhum.2022.815759. eCollection 2022. Front Hum Neurosci. 2022. PMID: 35845248 Free PMC article. Review.
-
Shortage of Cellular ATP as a Cause of Diseases and Strategies to Enhance ATP.Front Pharmacol. 2019 Feb 19;10:98. doi: 10.3389/fphar.2019.00098. eCollection 2019. Front Pharmacol. 2019. PMID: 30837873 Free PMC article.
-
Clinical effects of chemical exposures on mitochondrial function.Toxicology. 2017 Nov 1;391:90-99. doi: 10.1016/j.tox.2017.07.009. Epub 2017 Jul 27. Toxicology. 2017. PMID: 28757096 Free PMC article. Review.
References
-
- Lewis MR, Lewis WH. MITOCHONDRIA IN TISSUE CULTURE. Science (New York, NY) 1914;39:330–333. - PubMed
-
- Mitchell P, Moyle J. Chemiosmotic hypothesis of oxidative phosphorylation. Nature. 1967;213:137–139. - PubMed
-
- Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972;20:145–147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

