Globozoospermia and lack of acrosome formation in GM130-deficient mice
- PMID: 28055014
- PMCID: PMC5386352
- DOI: 10.1038/cddis.2016.414
Globozoospermia and lack of acrosome formation in GM130-deficient mice
Abstract
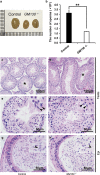
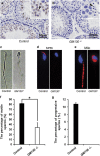
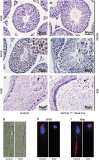
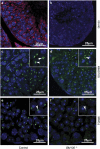
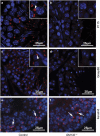
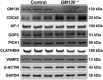
Globozoospermia is a common reproductive disorder that causes male infertility in humans, and the malformation or loss of acrosomes is the prominent feature of this disease. Although the acrosome is thought to be derived from the Golgi apparatus, the detailed molecular mechanisms remain unclear. GM130 is a cis-side localized Golgi matrix protein,whereas the physiological functions of this protein remain elusive. Here we showed that inactivation of GM130-caused male infertility in mouse model. The primary defects were the absence of acrosomes, round sperm heads, and aberrant assembly of the mitochondrial sheath, which comprise the characteristic features of human globozoospermia. Further investigation indicated that loss of GM130 did not affect the secretion of pro-acrosomic vesicles, whereas the vesicles failed to fuse into a single large acrosome vesicle. Co-localization of the adaptor protein complex AP1 and trans-Golgi network (TGN) protein TGN46 was disrupted, suggesting that the malformation of acrosomes is most likely due to the defect in the sorting and coating of Golgi-derived pro-acrosomic vesicles. Thus, the GM130-deficient mouse provides a valuable model for investigating the etiology of human globozoospermia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Loss of the Na+/H+ exchanger NHE8 causes male infertility in mice by disrupting acrosome formation.J Biol Chem. 2017 Jun 30;292(26):10845-10854. doi: 10.1074/jbc.M117.784108. Epub 2017 May 5. J Biol Chem. 2017. PMID: 28476888 Free PMC article.
-
PICK1 deficiency causes male infertility in mice by disrupting acrosome formation.J Clin Invest. 2009 Apr;119(4):802-12. doi: 10.1172/JCI36230. Epub 2009 Mar 2. J Clin Invest. 2009. PMID: 19258705 Free PMC article.
-
Lack of acrosome formation in mice lacking a Golgi protein, GOPC.Proc Natl Acad Sci U S A. 2002 Aug 20;99(17):11211-6. doi: 10.1073/pnas.162027899. Epub 2002 Jul 30. Proc Natl Acad Sci U S A. 2002. PMID: 12149515 Free PMC article.
-
Human globozoospermia-related genes and their role in acrosome biogenesis.WIREs Mech Dis. 2023 Mar;15(2):e1589. doi: 10.1002/wsbm.1589. Epub 2022 Dec 9. WIREs Mech Dis. 2023. PMID: 36493758 Review.
-
Molecular cytogenetic and genetic aspects of globozoospermia: a review.Andrologia. 2013 Feb;45(1):1-9. doi: 10.1111/j.1439-0272.2012.01308.x. Epub 2012 May 10. Andrologia. 2013. PMID: 22571172 Review.
Cited by
-
AU040320 deficiency leads to disruption of acrosome biogenesis and infertility in homozygous mutant mice.Sci Rep. 2018 Jul 10;8(1):10379. doi: 10.1038/s41598-018-28666-6. Sci Rep. 2018. PMID: 29991750 Free PMC article.
-
Sperm Morphology Assessment in the Era of Intracytoplasmic Sperm Injection: Reliable Results Require Focus on Standardization, Quality Control, and Training.World J Mens Health. 2022 Jul;40(3):347-360. doi: 10.5534/wjmh.210054. Epub 2021 Jun 17. World J Mens Health. 2022. PMID: 34169687 Free PMC article. Review.
-
Male reproductive phenotypes of genetically altered laboratory mice (Mus musculus): a review based on pertinent literature from the last three decades.Front Vet Sci. 2024 Mar 4;11:1272757. doi: 10.3389/fvets.2024.1272757. eCollection 2024. Front Vet Sci. 2024. PMID: 38500604 Free PMC article. Review.
-
Sperm acrosome overgrowth and infertility in mice lacking chromosome 18 pachytene piRNA.PLoS Genet. 2021 Apr 8;17(4):e1009485. doi: 10.1371/journal.pgen.1009485. eCollection 2021 Apr. PLoS Genet. 2021. PMID: 33831001 Free PMC article.
-
MIWI arginines orchestrate generation of functional pachytene piRNAs and spermiogenesis.bioRxiv [Preprint]. 2024 Jan 1:2023.12.31.573779. doi: 10.1101/2023.12.31.573779. bioRxiv. 2024. Update in: Nucleic Acids Res. 2024 Jun 24;52(11):6558-6570. doi: 10.1093/nar/gkae193. PMID: 38260298 Free PMC article. Updated. Preprint.
References
-
- Abou-Haila A, Tulsiani DR. Mammalian sperm acrosome: formation, contents, and function. Arch Biochem Biophys 2000; 379: 173–182. - PubMed
-
- Kullander S, Rausing A. On round-headed human spermatozoa. Int J Fertil 1975; 20: 33–40. - PubMed
-
- Lalonde L, Langlais J, Antaki P, Chapdelaine A, Roberts KD, Bleau G. Male infertility associated with round-headed acrosomeless spermatozoa. Fertil Steril 1988; 49: 316–321. - PubMed
-
- Singh G. Ultrastructural features of round-headed human spermatozoa. Int J Fertil 1992; 37: 99–102. - PubMed
-
- Battaglia DE, Koehler JK, Klein NA, Tucker MJ. Failure of oocyte activation after intracytoplasmic sperm injection using round-headed sperm. Fertil Steril 1997; 68: 118–122. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

