Epstein-Barr Virus: Diseases Linked to Infection and Transformation
- PMID: 27826287
- PMCID: PMC5078142
- DOI: 10.3389/fmicb.2016.01602
Epstein-Barr Virus: Diseases Linked to Infection and Transformation
Abstract
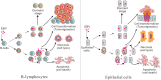
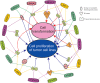
Epstein-Barr virus (EBV) was first discovered in 1964, and was the first known human tumor virus now shown to be associated with a vast number of human diseases. Numerous studies have been conducted to understand infection, propagation, and transformation in various cell types linked to human diseases. However, a comprehensive lens through which virus infection, reactivation and transformation of infected host cells can be visualized is yet to be formally established and will need much further investigation. Several human cell types infected by EBV have been linked to associated diseases. However, whether these are a direct result of EBV infection or indirectly due to contributions by additional infectious agents will need to be fully investigated. Therefore, a thorough examination of infection, reactivation, and cell transformation induced by EBV will provide a more detailed view of its contributions that drive pathogenesis. This undoubtedly expand our knowledge of the biology of EBV infection and the signaling activities of targeted cellular factors dysregulated on infection. Furthermore, these insights may lead to identification of therapeutic targets and agents for clinical interventions. Here, we review the spectrum of EBV-associated diseases, the role of the encoded latent antigens, and the switch to latency or lytic replication which occurs in EBV infected cells. Furthermore, we describe the cellular processes and critical factors which contribute to cell transformation. We also describe the fate of B-cells and epithelial cells after EBV infection and the expected consequences which contribute to establishment of viral-associated pathologies.
Keywords: B-cell; EBV; cancer; epithelial cell; infection; latency; lytic; transformation.
Figures
Similar articles
-
Cellular differentiation regulator BLIMP1 induces Epstein-Barr virus lytic reactivation in epithelial and B cells by activating transcription from both the R and Z promoters.J Virol. 2015 Feb;89(3):1731-43. doi: 10.1128/JVI.02781-14. Epub 2014 Nov 19. J Virol. 2015. PMID: 25410866 Free PMC article.
-
EBV Infection and Glucose Metabolism in Nasopharyngeal Carcinoma.Adv Exp Med Biol. 2017;1018:75-90. doi: 10.1007/978-981-10-5765-6_6. Adv Exp Med Biol. 2017. PMID: 29052133 Review.
-
Regulation and dysregulation of Epstein-Barr virus latency: implications for the development of autoimmune diseases.Autoimmunity. 2008 May;41(4):298-328. doi: 10.1080/08916930802024772. Autoimmunity. 2008. PMID: 18432410 Review.
-
Epstein-Barr virus latency: LMP2, a regulator or means for Epstein-Barr virus persistence?Adv Cancer Res. 2000;79:175-200. doi: 10.1016/s0065-230x(00)79006-3. Adv Cancer Res. 2000. PMID: 10818681 Review.
-
Inhibition of Epstein-Barr Virus Replication in Human Papillomavirus-Immortalized Keratinocytes.J Virol. 2019 Jan 4;93(2):e01216-18. doi: 10.1128/JVI.01216-18. Print 2019 Jan 15. J Virol. 2019. PMID: 30381489 Free PMC article.
Cited by
-
Cellular expression profiles of Epstein-Barr virus-transformed B-lymphoblastoid cell lines.Biomed Rep. 2020 Nov;13(5):43. doi: 10.3892/br.2020.1350. Epub 2020 Aug 27. Biomed Rep. 2020. PMID: 32934816 Free PMC article.
-
BHRF1, a BCL2 viral homolog, disturbs mitochondrial dynamics and stimulates mitophagy to dampen type I IFN induction.Autophagy. 2021 Jun;17(6):1296-1315. doi: 10.1080/15548627.2020.1758416. Epub 2020 May 13. Autophagy. 2021. PMID: 32401605 Free PMC article.
-
CRISPR-Cas9 Genetic Analysis of Virus-Host Interactions.Viruses. 2018 Jan 30;10(2):55. doi: 10.3390/v10020055. Viruses. 2018. PMID: 29385696 Free PMC article. Review.
-
Current Progress in EBV-Associated B-Cell Lymphomas.Adv Exp Med Biol. 2017;1018:57-74. doi: 10.1007/978-981-10-5765-6_5. Adv Exp Med Biol. 2017. PMID: 29052132 Free PMC article. Review.
-
The Function and Therapeutic Potential of Epstein-Barr Virus-Encoded MicroRNAs in Cancer.Mol Ther Nucleic Acids. 2019 Sep 6;17:657-668. doi: 10.1016/j.omtn.2019.07.002. Epub 2019 Jul 15. Mol Ther Nucleic Acids. 2019. PMID: 31400608 Free PMC article. Review.
References
-
- Accardi R., Fathallah I., Gruffat H., Mariggio G., Le Calvez-Kelm F., Voegele C., et al. (2013). Epstein – Barr virus transforming protein LMP-1 alters B cells gene expression by promoting accumulation of the oncoprotein DeltaNp73alpha. PLoS Pathog. 9:e1003186 10.1371/journal.ppat.1003186 - DOI - PMC - PubMed
-
- Adamson A. L., Darr D., Holley-Guthrie E., Johnson R. A., Mauser A., Swenson J., et al. (2000). Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing the levels of phosphorylated p38 and c-Jun N-terminal kinases. J. Virol. 74 1224–1233. 10.1128/JVI.74.3.1224-1233.2000 - DOI - PMC - PubMed
-
- Alexander F. E., Lawrence D. J., Freeland J., Krajewski A. S., Angus B., Taylor G. M., et al. (2003). An epidemiologic study of index and family infectious mononucleosis and adult Hodgkin’s disease (HD): evidence for a specific association with EBV+ve HD in young adults. Int. J. Cancer 107 298–302. 10.1002/ijc.11156 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

