Nuclear respiratory factor 1 and endurance exercise promote human telomere transcription
- PMID: 27819056
- PMCID: PMC5087959
- DOI: 10.1126/sciadv.1600031
Nuclear respiratory factor 1 and endurance exercise promote human telomere transcription
Abstract
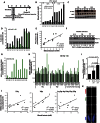
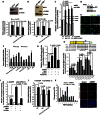
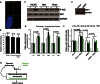
DNA breaks activate the DNA damage response and, if left unrepaired, trigger cellular senescence. Telomeres are specialized nucleoprotein structures that protect chromosome ends from persistent DNA damage response activation. Whether protection can be enhanced to counteract the age-dependent decline in telomere integrity is a challenging question. Telomeric repeat-containing RNA (TERRA), which is transcribed from telomeres, emerged as important player in telomere integrity. However, how human telomere transcription is regulated is still largely unknown. We identify nuclear respiratory factor 1 and peroxisome proliferator-activated receptor γ coactivator 1α as regulators of human telomere transcription. In agreement with an upstream regulation of these factors by adenosine 5'-monophosphate (AMP)-activated protein kinase (AMPK), pharmacological activation of AMPK in cancer cell lines or in normal nonproliferating myotubes up-regulated TERRA, thereby linking metabolism to telomere fitness. Cycling endurance exercise, which is associated with AMPK activation, increased TERRA levels in skeletal muscle biopsies obtained from 10 healthy young volunteers. The data support the idea that exercise may protect against aging.
Keywords: AMPK; NRF1; TERRA; Telomere; exercise.
Figures

Similar articles
-
Repression of TERRA Expression by Subtelomeric DNA Methylation Is Dependent on NRF1 Binding.Int J Mol Sci. 2019 Jun 7;20(11):2791. doi: 10.3390/ijms20112791. Int J Mol Sci. 2019. PMID: 31181625 Free PMC article.
-
Effects of alpha-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle.FASEB J. 2005 Jul;19(9):1146-8. doi: 10.1096/fj.04-3144fje. Epub 2005 May 5. FASEB J. 2005. PMID: 15878932
-
Sensing and responding to energetic stress: The role of the AMPK-PGC1α-NRF1 axis in control of mitochondrial biogenesis in fish.Comp Biochem Physiol B Biochem Mol Biol. 2016 Sep;199:4-12. doi: 10.1016/j.cbpb.2015.09.005. Epub 2015 Sep 21. Comp Biochem Physiol B Biochem Mol Biol. 2016. PMID: 26393435
-
Telomeric noncoding RNA: telomeric repeat-containing RNA in telomere biology.Wiley Interdiscip Rev RNA. 2014 May-Jun;5(3):407-19. doi: 10.1002/wrna.1220. Epub 2014 Feb 12. Wiley Interdiscip Rev RNA. 2014. PMID: 24523222 Review.
-
Telomeric repeat-containing RNA TERRA: a noncoding RNA connecting telomere biology to genome integrity.Front Genet. 2015 Apr 14;6:143. doi: 10.3389/fgene.2015.00143. eCollection 2015. Front Genet. 2015. PMID: 25926849 Free PMC article. Review.
Cited by
-
Can Antidiabetic Medications Affect Telomere Length in Patients with Type 2 Diabetes? A Mini-Review.Diabetes Metab Syndr Obes. 2023 Nov 22;16:3739-3750. doi: 10.2147/DMSO.S428560. eCollection 2023. Diabetes Metab Syndr Obes. 2023. PMID: 38028989 Free PMC article. Review.
-
Metformin and insulin treatment prevent placental telomere attrition in boys exposed to maternal diabetes.PLoS One. 2018 Dec 11;13(12):e0208533. doi: 10.1371/journal.pone.0208533. eCollection 2018. PLoS One. 2018. PMID: 30533028 Free PMC article.
-
Physical Activity and Nutrition: Two Promising Strategies for Telomere Maintenance?Nutrients. 2018 Dec 7;10(12):1942. doi: 10.3390/nu10121942. Nutrients. 2018. PMID: 30544511 Free PMC article. Review.
-
Exosc9 Initiates SUMO-Dependent lncRNA TERRA Degradation to Impact Telomeric Integrity in Endocrine Therapy Insensitive Hormone Receptor-Positive Breast Cancer.Cells. 2023 Oct 20;12(20):2495. doi: 10.3390/cells12202495. Cells. 2023. PMID: 37887339 Free PMC article.
-
Snail1 transcription factor controls telomere transcription and integrity.Nucleic Acids Res. 2018 Jan 9;46(1):146-158. doi: 10.1093/nar/gkx958. Nucleic Acids Res. 2018. PMID: 29059385 Free PMC article.
References
-
- Blackburn E. H., Epel E. S., Lin J., Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 350, 1193–1198 (2015). - PubMed
-
- Azzalin C. M., Reichenbach P., Khoriauli L., Giulotto E., Lingner J., Telomeric repeat–containing RNA and RNA surveillance factors at mammalian chromosome ends. Science 318, 798–801 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

