In Vivo Role of Neutrophil Extracellular Traps in Antiphospholipid Antibody-Mediated Venous Thrombosis
- PMID: 27696751
- PMCID: PMC5329054
- DOI: 10.1002/art.39938
In Vivo Role of Neutrophil Extracellular Traps in Antiphospholipid Antibody-Mediated Venous Thrombosis
Abstract
Objective: Antiphospholipid syndrome (APS) is a leading acquired cause of thrombotic events. Although antiphospholipid antibodies have been shown to promote thrombosis in mice, the role of neutrophils has not been explicitly studied. The aim of this study was to characterize neutrophils in the context of a new model of antiphospholipid antibody-mediated venous thrombosis.
Methods: Mice were administered fractions of IgG obtained from patients with APS. At the same time, blood flow through the inferior vena cava was reduced by induction of stenosis. Resulting thrombi were characterized for size and neutrophil content. Circulating factors and the vessel wall were also assessed.
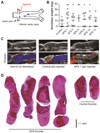
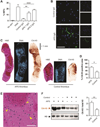
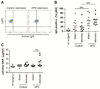
Results: As measured by both thrombus weight and thrombosis frequency, mice treated with IgG from patients with APS (APS IgG) demonstrated exaggerated thrombosis as compared with control IgG-treated mice. Thrombi in mice treated with APS IgG were enriched for citrullinated histone H3 (a marker of neutrophil extracellular traps [NETs]). APS IgG-treated mice also demonstrated elevated levels of circulating cell-free DNA and human IgG bound to the neutrophil surface. In contrast, circulating neutrophil numbers and markers of vessel wall activation were not appreciably different between APS IgG-treated mice and control mice. Treatment with either DNase (which dissolves NETs) or a neutrophil-depleting antibody reduced thrombosis in APS IgG-treated mice to the level in control mice.
Conclusion: These data support a mechanism whereby circulating neutrophils are primed by antiphospholipid antibodies to accelerate thrombosis. This line of investigation suggests new, immunomodulatory approaches for the treatment of APS.
© 2016, American College of Rheumatology.
Conflict of interest statement
The authors have no competing interests or conflicts to disclose.
Figures



Similar articles
-
Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: a newly identified mechanism of thrombosis in the antiphospholipid syndrome.Arthritis Rheumatol. 2015 Nov;67(11):2990-3003. doi: 10.1002/art.39247. Arthritis Rheumatol. 2015. PMID: 26097119 Free PMC article.
-
Activated signature of antiphospholipid syndrome neutrophils reveals potential therapeutic target.JCI Insight. 2017 Sep 21;2(18):e93897. doi: 10.1172/jci.insight.93897. eCollection 2017 Sep 21. JCI Insight. 2017. PMID: 28931754 Free PMC article.
-
Cofactor-independent human antiphospholipid antibodies induce venous thrombosis in mice.J Thromb Haemost. 2016 May;14(5):1011-20. doi: 10.1111/jth.13263. Epub 2016 Mar 16. J Thromb Haemost. 2016. PMID: 26786324
-
Biological markers of high risk of thrombotic recurrence in patients with antiphospholipid syndrome: A literature review.Autoimmun Rev. 2024 Jun;23(6):103585. doi: 10.1016/j.autrev.2024.103585. Epub 2024 Jul 31. Autoimmun Rev. 2024. PMID: 39094811 Review.
-
Probing antiphospholipid-mediated thrombosis: the interplay between anticardiolipin antibodies and endothelial cells.Lupus. 2003;12(7):539-45. doi: 10.1191/961203303lu398oa. Lupus. 2003. PMID: 12892395 Review.
Cited by
-
Therapeutic Potential and Immunomodulatory Role of Coenzyme Q10 and Its Analogues in Systemic Autoimmune Diseases.Antioxidants (Basel). 2021 Apr 13;10(4):600. doi: 10.3390/antiox10040600. Antioxidants (Basel). 2021. PMID: 33924642 Free PMC article. Review.
-
Parvovirus B19 Infection Is Associated with the Formation of Neutrophil Extracellular Traps and Thrombosis: A Possible Linkage of the VP1 Unique Region.Int J Mol Sci. 2024 Sep 13;25(18):9917. doi: 10.3390/ijms25189917. Int J Mol Sci. 2024. PMID: 39337405 Free PMC article.
-
Chloroquine reduces hypercoagulability in pancreatic cancer through inhibition of neutrophil extracellular traps.BMC Cancer. 2018 Jun 22;18(1):678. doi: 10.1186/s12885-018-4584-2. BMC Cancer. 2018. PMID: 29929491 Free PMC article.
-
Podoplanin promotes tumor growth, platelet aggregation, and venous thrombosis in murine models of ovarian cancer.J Thromb Haemost. 2022 Jan;20(1):104-114. doi: 10.1111/jth.15544. Epub 2021 Oct 15. J Thromb Haemost. 2022. PMID: 34608736 Free PMC article.
-
A 37-Year-Old Man With Primary Antiphospholipid Syndrome Presenting With Respiratory Distress and Worsening Toe Ischemia.Arthritis Care Res (Hoboken). 2017 Aug;69(8):1253-1259. doi: 10.1002/acr.23168. Arthritis Care Res (Hoboken). 2017. PMID: 27992694 Free PMC article. No abstract available.
References
-
- Gomez-Puerta JA, Cervera R. Diagnosis and classification of the antiphospholipid syndrome. Journal of autoimmunity. 2014;48–49:20–25. - PubMed
-
- Bertolaccini ML, Amengual O, Andreoli L, Atsumi T, Chighizola CB, Forastiero R, et al. 14th International Congress on Antiphospholipid Antibodies Task Force. Report on antiphospholipid syndrome laboratory diagnostics and trends. Autoimmunity reviews. 2014;13(9):917–930. - PubMed
-
- Abreu MM, Danowski A, Wahl DG, Amigo MC, Tektonidou M, Pacheco MS, et al. The relevance of "non-criteria" clinical manifestations of antiphospholipid syndrome: 14th International Congress on Antiphospholipid Antibodies Technical Task Force Report on Antiphospholipid Syndrome Clinical Features. Autoimmunity reviews. 2015;14(5):401–414. - PubMed
-
- Willis R, Harris EN, Pierangeli SS. Pathogenesis of the antiphospholipid syndrome. Seminars in thrombosis and hemostasis. 2012;38(4):305–321. - PubMed
-
- Pericleous C, Ruiz-Limon P, Romay-Penabad Z, Marin AC, Garza-Garcia A, Murfitt L, et al. Proof-of-concept study demonstrating the pathogenicity of affinity-purified IgG antibodies directed to domain I of beta2-glycoprotein I in a mouse model of anti-phospholipid antibody-induced thrombosis. Rheumatology. 2015;54(4):722–727. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

