Antitumor Activity of Pembrolizumab in Biomarker-Unselected Patients With Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Results From the Phase Ib KEYNOTE-012 Expansion Cohort
- PMID: 27646946
- PMCID: PMC6804896
- DOI: 10.1200/JCO.2016.68.1478
Antitumor Activity of Pembrolizumab in Biomarker-Unselected Patients With Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Results From the Phase Ib KEYNOTE-012 Expansion Cohort
Abstract
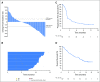
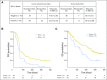
Purpose Treatment with pembrolizumab, an anti-programmed death-1 antibody, at 10 mg/kg administered once every 2 weeks, displayed durable antitumor activity in programmed death-ligand 1 (PD-L1) -positive recurrent and/or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC) in the KEYNOTE-012 trial. Results from the expansion cohort, in which patients with HNSCC, irrespective of biomarker status, received a fixed dose of pembrolizumab at a less frequent dosing schedule, are reported. Patients and Methods Patients with R/M HNSCC, irrespective of PD-L1 or human papillomavirus status, received pembrolizumab 200 mg intravenously once every 3 weeks. Imaging was performed every 8 weeks. Primary end points were overall response rate (ORR) per central imaging vendor (Response Evaluation Criteria in Solid Tumors v1.1) and safety. Secondary end points included progression-free survival, overall survival, and association of response and PD-L1 expression. Patients who received one or more doses of pembrolizumab were included in analyses. Results Of 132 patients enrolled, median age was 60 years (range, 25 to 84 years), 83% were male, and 57% received two or more lines of therapy for R/M disease. ORR was 18% (95% CI, 12 to 26) by central imaging vendor and 20% (95% CI, 13 to 28) by investigator review. Median duration of response was not reached (range, ≥ 2 to ≥ 11 months). Six-month progression-free survival and overall survival rates were 23% and 59%, respectively. By using tumor and immune cells, a statistically significant increase in ORR was observed for PD-L1-positive versus -negative patients (22% v 4%; P = .021). Treatment-related adverse events of any grade and grade ≥ 3 events occurred in 62% and 9% of patients, respectively. Conclusion Fixed-dose pembrolizumab 200 mg administered once every 3 weeks was well tolerated and yielded a clinically meaningful ORR with evidence of durable responses, which supports further development of this regimen in patients with advanced HNSCC.
Trial registration: ClinicalTrials.gov NCT01848834.
Conflict of interest statement
Authors’ disclosures of potential conflicts of interest are found in the article online at
Figures
Comment in
-
Immune Checkpoint Inhibition in Cancers that Affect the Head and Neck.Int J Radiat Oncol Biol Phys. 2017 Aug 1;98(5):969-973. doi: 10.1016/j.ijrobp.2017.03.003. Epub 2017 Jul 10. Int J Radiat Oncol Biol Phys. 2017. PMID: 28721906 No abstract available.
Similar articles
-
Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial.Lancet Oncol. 2016 Jul;17(7):956-965. doi: 10.1016/S1470-2045(16)30066-3. Epub 2016 May 27. Lancet Oncol. 2016. PMID: 27247226 Clinical Trial.
-
Safety and Antitumor Activity of Pembrolizumab in Patients With Programmed Death-Ligand 1-Positive Nasopharyngeal Carcinoma: Results of the KEYNOTE-028 Study.J Clin Oncol. 2017 Dec 20;35(36):4050-4056. doi: 10.1200/JCO.2017.73.3675. Epub 2017 Aug 24. J Clin Oncol. 2017. PMID: 28837405 Clinical Trial.
-
Safety and Antitumor Activity of the Anti-Programmed Death-1 Antibody Pembrolizumab in Patients With Advanced Esophageal Carcinoma.J Clin Oncol. 2018 Jan 1;36(1):61-67. doi: 10.1200/JCO.2017.74.9846. Epub 2017 Nov 8. J Clin Oncol. 2018. PMID: 29116900 Clinical Trial.
-
Immunotherapy in recurrent and or metastatic squamous cell carcinoma of the head and neck.Curr Opin Oncol. 2019 May;31(3):146-151. doi: 10.1097/CCO.0000000000000522. Curr Opin Oncol. 2019. PMID: 30893146 Review.
-
Pembrolizumab and its use in the treatment of recurrent or metastatic head and neck cancer.Future Oncol. 2018 Jul;14(16):1547-1558. doi: 10.2217/fon-2017-0628. Epub 2018 Feb 21. Future Oncol. 2018. PMID: 29464975 Review.
Cited by
-
Immune-related gene TM4SF18 could promote the metastasis of gastric cancer cells and predict the prognosis of gastric cancer patients.Mol Oncol. 2022 Dec;16(22):4043-4059. doi: 10.1002/1878-0261.13321. Epub 2022 Oct 20. Mol Oncol. 2022. PMID: 36209368 Free PMC article.
-
Improved efficacy of pembrolizumab combined with soluble EphB4-albumin in HPV-negative EphrinB2 positive head neck squamous cell carcinoma.Oncotarget. 2024 Jul 10;15:444-458. doi: 10.18632/oncotarget.28605. Oncotarget. 2024. PMID: 38985143 Free PMC article. Clinical Trial.
-
HSPA12B Secreted by Tumor-Associated Endothelial Cells Might Induce M2 Polarization of Macrophages via Activating PI3K/Akt/mTOR Signaling.Onco Targets Ther. 2020 Sep 11;13:9103-9111. doi: 10.2147/OTT.S254985. eCollection 2020. Onco Targets Ther. 2020. PMID: 32982299 Free PMC article.
-
Treatment-Related Serious Adverse Events of Immune Checkpoint Inhibitors in Clinical Trials: A Systematic Review.Front Oncol. 2021 May 11;11:621639. doi: 10.3389/fonc.2021.621639. eCollection 2021. Front Oncol. 2021. PMID: 34046338 Free PMC article.
-
Efficient Delivery and Replication of Oncolytic Virus for Successful Treatment of Head and Neck Cancer.Int J Mol Sci. 2020 Sep 25;21(19):7073. doi: 10.3390/ijms21197073. Int J Mol Sci. 2020. PMID: 32992948 Free PMC article. Review.
References
-
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. - PubMed
-
- Cohen EE, LaMonte SJ, Erb NL, et al. American Cancer Society head and neck cancer survivorship care guideline. CA Cancer J Clin. 2016;66:203–239. - PubMed
-
- Denaro N, Russi EG, Adamo V, et al. State-of-the-art and emerging treatment options in the management of head and neck cancer: News from 2013. Oncology. 2014;86:212–229. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

