Cardiac myosin-Th17 responses promote heart failure in human myocarditis
- PMID: 27366791
- PMCID: PMC4924810
- DOI: 10.1172/jci.insight.85851
Cardiac myosin-Th17 responses promote heart failure in human myocarditis
Abstract
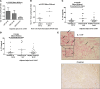
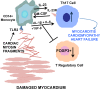
In human myocarditis and its sequela dilated cardiomyopathy (DCM), the mechanisms and immune phenotype governing disease and subsequent heart failure are not known. Here, we identified a Th17 cell immunophenotype of human myocarditis/DCM with elevated CD4+IL17+ T cells and Th17-promoting cytokines IL-6, TGF-β, and IL-23 as well as GM-CSF-secreting CD4+ T cells. The Th17 phenotype was linked with the effects of cardiac myosin on CD14+ monocytes, TLR2, and heart failure. Persistent heart failure was associated with high percentages of IL-17-producing T cells and IL-17-promoting cytokines, and the myocarditis/DCM phenotype included significantly low percentages of FOXP3+ Tregs, which may contribute to disease severity. We demonstrate a potentially novel mechanism in human myocarditis/DCM in which TLR2 peptide ligands from human cardiac myosin stimulated exaggerated Th17-related cytokines including TGF-β, IL-6, and IL-23 from myocarditic CD14+ monocytes in vitro, and an anti-TLR2 antibody abrogated the cytokine response. Our translational study explains how an immune phenotype may be initiated by cardiac myosin TLR ligand stimulation of monocytes to generate Th17-promoting cytokines and development of pathogenic Th17 cells in human myocarditis and heart failure, and provides a rationale for targeting IL-17A as a therapeutic option.
Figures








Similar articles
-
Interleukin-17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy.Circ Res. 2010 May 28;106(10):1646-55. doi: 10.1161/CIRCRESAHA.109.213157. Epub 2010 Apr 8. Circ Res. 2010. PMID: 20378858
-
Inhibition of microRNA-155 ameliorates experimental autoimmune myocarditis by modulating Th17/Treg immune response.J Mol Med (Berl). 2016 Sep;94(9):1063-79. doi: 10.1007/s00109-016-1414-3. Epub 2016 Apr 6. J Mol Med (Berl). 2016. PMID: 27052830
-
Myocarditis Elicits Dendritic Cell and Monocyte Infiltration in the Heart and Self-Antigen Presentation by Conventional Type 2 Dendritic Cells.Front Immunol. 2018 Nov 21;9:2714. doi: 10.3389/fimmu.2018.02714. eCollection 2018. Front Immunol. 2018. PMID: 30524444 Free PMC article.
-
Cardiac autoantibodies to myosin and other heart-specific autoantigens in myocarditis and dilated cardiomyopathy.Autoimmunity. 2001;34(3):199-204. doi: 10.3109/08916930109007385. Autoimmunity. 2001. PMID: 11908778 Review.
-
Circulating cardiac autoantibodies in dilated cardiomyopathy and myocarditis: pathogenetic and clinical significance.Eur J Heart Fail. 2002 Aug;4(4):411-7. doi: 10.1016/s1388-9842(02)00010-7. Eur J Heart Fail. 2002. PMID: 12167378 Review.
Cited by
-
Myocarditis and inflammatory cardiomyopathy: current evidence and future directions.Nat Rev Cardiol. 2021 Mar;18(3):169-193. doi: 10.1038/s41569-020-00435-x. Epub 2020 Oct 12. Nat Rev Cardiol. 2021. PMID: 33046850 Free PMC article. Review.
-
Sex differences in left-ventricular strain in a murine model of coxsackievirus B3 myocarditis.iScience. 2023 Nov 20;26(12):108493. doi: 10.1016/j.isci.2023.108493. eCollection 2023 Dec 15. iScience. 2023. PMID: 38146431 Free PMC article.
-
Cardiac myosin-specific autoimmune T cells contribute to immune-checkpoint-inhibitor-associated myocarditis.Cell Rep. 2022 Nov 8;41(6):111611. doi: 10.1016/j.celrep.2022.111611. Cell Rep. 2022. PMID: 36351411 Free PMC article.
-
Sex Differences, Genetic and Environmental Influences on Dilated Cardiomyopathy.J Clin Med. 2021 May 25;10(11):2289. doi: 10.3390/jcm10112289. J Clin Med. 2021. PMID: 34070351 Free PMC article. Review.
-
Circulating c-Met-Expressing Memory T Cells Define Cardiac Autoimmunity.Circulation. 2022 Dec 20;146(25):1930-1945. doi: 10.1161/CIRCULATIONAHA.121.055610. Epub 2022 Nov 23. Circulation. 2022. PMID: 36417924 Free PMC article.
References
-
- Neu N, Beisel KW, Traystman MD, Rose NR, Craig SW. Autoantibodies specific for the cardiac myosin isoform are found in mice susceptible to coxsackievirus B3 induced myocarditis. J Immunol. 1987;138(8):2488–2492. - PubMed
-
- Caforio AL, Mahon NG, McKenna WJ. Clinical implications of anti-cardiac immunity in dilated cardiomyopathy. Ernst Schering Res Found Workshop. 2006;(55):169–193. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

