Prognostic significance of PLIN1 expression in human breast cancer
- PMID: 27359054
- PMCID: PMC5342357
- DOI: 10.18632/oncotarget.10239
Prognostic significance of PLIN1 expression in human breast cancer
Abstract
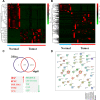
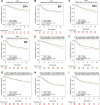
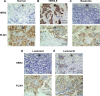
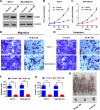
Breast cancer is a heterogeneous disease associated with diverse clinical, biological and molecular features, presenting huge challenges for prognosis and treatment. Here we found that perilipin-1 (PLIN1) mRNA expression is significantly downregulated in human breast cancer. Kaplan-Meier analysis indicated that patients presenting with reduced PLIN1 expression exhibited poorer overall metastatic relapse-free survival (p = 0.03). Further Cox proportional hazard models analysis revealed that the reduced expression of PLIN1 is an independent predictor of overall survival in estrogen receptor positive (p < 0.0001, HR = 0.87, 95% CI = 0.81-0.92, N = 3,600) and luminal A-subtype (p = 0.02, HR = 0.88, 95% CI = 0.78-0.98, N = 1,469) breast cancer patients. We also demonstrated that the exogenous expression of PLIN1 in human breast cancer MCF-7 and MDA-MB-231 cells significantly inhibits cell proliferation, migration, invasion and in vivo tumorigenesis in mice. Together, these data provide novel insights into a prognostic significance of PLIN1 in human breast cancer and reveal a potentially new gene therapy target for breast cancer.
Keywords: Kaplan-Meier analysis; biomarker; meta analysis; perilipin-1; tumor suppressor.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures

Similar articles
-
Redefining prognostic factors for breast cancer: YB-1 is a stronger predictor of relapse and disease-specific survival than estrogen receptor or HER-2 across all tumor subtypes.Breast Cancer Res. 2008;10(5):R86. doi: 10.1186/bcr2156. Epub 2008 Oct 16. Breast Cancer Res. 2008. PMID: 18925950 Free PMC article.
-
Prognostic significance of interferon regulating factor 4 (IRF4) in node-negative breast cancer.J Cancer Res Clin Oncol. 2017 Jul;143(7):1123-1131. doi: 10.1007/s00432-017-2377-7. Epub 2017 Mar 1. J Cancer Res Clin Oncol. 2017. PMID: 28251349
-
Human mitochondrial pyrroline-5-carboxylate reductase 1 promotes invasiveness and impacts survival in breast cancers.Carcinogenesis. 2017 May 1;38(5):519-531. doi: 10.1093/carcin/bgx022. Carcinogenesis. 2017. PMID: 28379297
-
Prognostic value of phosphorylated HER2 in HER2-positive breast cancer patients treated with adjuvant trastuzumab.Breast Cancer. 2015 May;22(3):292-9. doi: 10.1007/s12282-013-0478-y. Epub 2013 Jun 8. Breast Cancer. 2015. PMID: 23749689
-
Biological markers for designing clinical protocols.Ann N Y Acad Sci. 1993 Nov 30;698:271-8. doi: 10.1111/j.1749-6632.1993.tb17218.x. Ann N Y Acad Sci. 1993. PMID: 8279767 Review. No abstract available.
Cited by
-
Study of Gene Expression Profiles of Breast Cancers in Indian Women.Sci Rep. 2019 Jul 10;9(1):10018. doi: 10.1038/s41598-019-46261-1. Sci Rep. 2019. PMID: 31292488 Free PMC article.
-
Autosurv: interpretable deep learning framework for cancer survival analysis incorporating clinical and multi-omics data.NPJ Precis Oncol. 2024 Jan 5;8(1):4. doi: 10.1038/s41698-023-00494-6. NPJ Precis Oncol. 2024. PMID: 38182734 Free PMC article.
-
Integral membrane protein 2A inhibits cell growth in human breast cancer via enhancing autophagy induction.Cell Commun Signal. 2019 Aug 22;17(1):105. doi: 10.1186/s12964-019-0422-7. Cell Commun Signal. 2019. PMID: 31438969 Free PMC article.
-
Pygopus2 inhibits the efficacy of paclitaxel-induced apoptosis and induces multidrug resistance in human glioma cells.Oncotarget. 2017 Apr 25;8(17):27915-27928. doi: 10.18632/oncotarget.15843. Oncotarget. 2017. PMID: 28427190 Free PMC article.
-
Cancer radioresistance is characterized by a differential lipid droplet content along the cell cycle.Cell Div. 2024 Apr 20;19(1):14. doi: 10.1186/s13008-024-00116-y. Cell Div. 2024. PMID: 38643120 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

