The K1 Protein of Kaposi's Sarcoma-Associated Herpesvirus Augments Viral Lytic Replication
- PMID: 27307571
- PMCID: PMC4988170
- DOI: 10.1128/JVI.03102-15
The K1 Protein of Kaposi's Sarcoma-Associated Herpesvirus Augments Viral Lytic Replication
Abstract
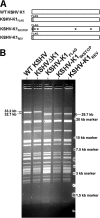
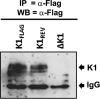
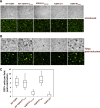
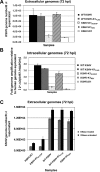
The K1 gene product of Kaposi's sarcoma-associated herpesvirus (KSHV) is encoded by the first open reading frame (ORF) of the viral genome. To investigate the role of the K1 gene during the KSHV life cycle, we constructed a set of recombinant viruses that contained either wild-type (WT) K1, a deleted K1 ORF (KSHVΔK1), stop codons within the K1 ORF (KSHV-K15×STOP), or a revertant K1 virus (KSHV-K1REV). We report that the recombinant viruses KSHVΔK1 and KSHV-K15×STOP displayed significantly reduced lytic replication compared to WT KSHV and KSHV-K1REV upon reactivation from latency. Additionally, cells infected with the recombinant viruses KSHVΔK1 and KSHV-K15×STOP also yielded smaller amounts of infectious progeny upon reactivation than did WT KSHV- and KSHV-K1REV-infected cells. Upon reactivation from latency, WT KSHV- and KSHV-K1REV-infected cells displayed activated Akt kinase, as evidenced by its phosphorylation, while cells infected with viruses deleted for K1 showed reduced phosphorylation and activation of Akt kinase. Overall, our results suggest that K1 plays an important role during the KSHV life cycle.
Importance: Kaposi's sarcoma-associated herpesvirus (KSHV) is the etiological agent of three human malignancies, and KSHV K1 is a signaling protein that has been shown to be involved in cellular transformation and to activate the phosphatidylinositol 3-kinase (PI3K)/Akt/mTOR pathway. In order to investigate the role of the K1 protein in the life cycle of KSHV, we constructed recombinant viruses that were deficient for K1. We found that K1 deletion viruses displayed reduced lytic replication compared to the WT virus and also yielded smaller numbers of infectious progeny. We report that K1 plays an important role in the life cycle of KSHV.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
Kaposi's Sarcoma-Associated Herpesvirus Nonstructural Membrane Protein pK15 Recruits the Class II Phosphatidylinositol 3-Kinase PI3K-C2α To Activate Productive Viral Replication.J Virol. 2018 Aug 16;92(17):e00544-18. doi: 10.1128/JVI.00544-18. Print 2018 Sep 1. J Virol. 2018. PMID: 29950425 Free PMC article.
-
The DNase Activity of Kaposi's Sarcoma-Associated Herpesvirus SOX Protein Serves an Important Role in Viral Genome Processing during Lytic Replication.J Virol. 2019 Apr 3;93(8):e01983-18. doi: 10.1128/JVI.01983-18. Print 2019 Apr 15. J Virol. 2019. PMID: 30728255 Free PMC article.
-
Suppression of tetradecanoyl phorbol acetate-induced lytic reactivation of Kaposi's sarcoma-associated herpesvirus by K1 signal transduction.J Virol. 2002 Dec;76(23):12185-99. doi: 10.1128/jvi.76.23.12185-12199.2002. J Virol. 2002. PMID: 12414958 Free PMC article.
-
[Replication Machinery of Kaposi's Sarcoma-associated Herpesvirus and Drug Discovery Research].Yakugaku Zasshi. 2019;139(1):69-73. doi: 10.1248/yakushi.18-00164-2. Yakugaku Zasshi. 2019. PMID: 30606932 Review. Japanese.
-
Regulation of KSHV Latency and Lytic Reactivation.Viruses. 2020 Sep 17;12(9):1034. doi: 10.3390/v12091034. Viruses. 2020. PMID: 32957532 Free PMC article. Review.
Cited by
-
Kaposi's Sarcoma-Associated Herpesvirus Nonstructural Membrane Protein pK15 Recruits the Class II Phosphatidylinositol 3-Kinase PI3K-C2α To Activate Productive Viral Replication.J Virol. 2018 Aug 16;92(17):e00544-18. doi: 10.1128/JVI.00544-18. Print 2018 Sep 1. J Virol. 2018. PMID: 29950425 Free PMC article.
-
Contribution of the KSHV and EBV lytic cycles to tumourigenesis.Curr Opin Virol. 2018 Oct;32:60-70. doi: 10.1016/j.coviro.2018.08.014. Epub 2018 Sep 28. Curr Opin Virol. 2018. PMID: 30268927 Free PMC article. Review.
-
Kaposi sarcoma herpesvirus pathogenesis.Philos Trans R Soc Lond B Biol Sci. 2017 Oct 19;372(1732):20160275. doi: 10.1098/rstb.2016.0275. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28893942 Free PMC article. Review.
-
Kaposi's Sarcoma-Associated Herpesvirus (KSHV)-Associated Disease in the AIDS Patient: An Update.Cancer Treat Res. 2019;177:63-80. doi: 10.1007/978-3-030-03502-0_3. Cancer Treat Res. 2019. PMID: 30523621 Free PMC article. Review.
-
Kaposi Sarcoma-Associated Herpesvirus Glycoprotein H Is Indispensable for Infection of Epithelial, Endothelial, and Fibroblast Cell Types.J Virol. 2019 Jul 30;93(16):e00630-19. doi: 10.1128/JVI.00630-19. Print 2019 Aug 15. J Virol. 2019. PMID: 31142670 Free PMC article.
References
-
- Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d'Agay MF, Clauvel JP, Raphael M, Degos L, Sigaux F. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276–1280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

