Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial
- PMID: 27283860
- PMCID: PMC4993103
- DOI: 10.1016/S1470-2045(16)30146-2
Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial
Abstract
Background: BRAF mutations act as an oncogenic driver via the mitogen-activated protein kinase (MAPK) pathway in non-small cell lung cancer (NSCLC). BRAF inhibition has shown antitumour activity in patients with BRAF(V600E)-mutant NSCLC. Dual MAPK pathway inhibition with BRAF and MEK inhibitors in BRAF(V600E)-mutant NSCLC might improve efficacy over BRAF inhibitor monotherapy based on observations in BRAF(V600)-mutant melanoma. We aimed to assess the antitumour activity and safety of dabrafenib plus trametinib in patients with BRAF(V600E)-mutant NSCLC.
Methods: In this phase 2, multicentre, non-randomised, open-label study, we enrolled adult patients (aged ≥18 years) with pretreated metastatic stage IV BRAF(V600E)-mutant NSCLC who had documented tumour progression after at least one previous platinum-based chemotherapy and had had no more than three previous systemic anticancer therapies. Patients with previous BRAF or MEK inhibitor treatment were ineligible. Patients with brain metastases were allowed to enrol only if the lesions were asymptomatic, untreated (or stable more than 3 weeks after local therapy if treated), and measured less than 1 cm. Enrolled patients received oral dabrafenib (150 mg twice daily) plus oral trametinib (2 mg once daily) in continuous 21-day cycles until disease progression, unacceptable adverse events, withdrawal of consent, or death. The primary endpoint was investigator-assessed overall response, which was assessed by intention to treat in the protocol-defined population (patients who received second-line or later treatment); safety was also assessed in this population and was assessed at least once every 3 weeks, with adverse events, laboratory values, and vital signs graded according to the Common Terminology Criteria for Adverse Events version 4.0. The study is ongoing but no longer recruiting patients. This trial is registered with ClinicalTrials.gov, number NCT01336634.
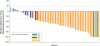
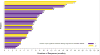
Findings: Between Dec 20, 2013, and Jan 14, 2015, 59 patients from 30 centres in nine countries across North America, Europe, and Asia met eligibility criteria. Two patients who had previously been untreated due to protocol deviation were excluded; thus, 57 eligible patients were enrolled. 36 patients (63·2% [95% CI 49·3-75·6]) achieved an investigator-assessed overall response. Serious adverse events were reported in 32 (56%) of 57 patients and included pyrexia in nine (16%), anaemia in three (5%), confusional state in two (4%), decreased appetite in two (4%), haemoptysis in two (4%), hypercalcaemia in two (4%), nausea in two (4%), and cutaneous squamous cell carcinoma in two (4%). The most common grade 3-4 adverse events were neutropenia in five patients (9%), hyponatraemia in four (7%), and anaemia in three (5%). Four patients died during the study from fatal adverse events judged to be unrelated to treatment (one retroperitoneal haemorrhage, one subarachnoid haemorrhage, one respiratory distress, and one from disease progression that was more severe than typical progression, as assessed by the investigator).
Interpretation: Dabrafenib plus trametinib could represent a new targeted therapy with robust antitumour activity and a manageable safety profile in patients with BRAF(V600E)-mutant NSCLC.
Funding: GlaxoSmithKline.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests DP acts as an advisor for AstraZeneca, Boehringer, Bristol-Myers Squibb, Clovis, Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Sanofi and has received research funding from Novartis unrelated to the current trial. BB’s institution received a grant from Novartis for this study. HJMG’s institution has received payments from Eli Lilly, Merck Sharp & Dohme, and Roche. P-JS has been involved in clinical trials for GlaxoSmithKline and Novartis. EQ has received personal fees from AbbVie, Bristol-Myers Squibb, Clovis, Lilly, Pfizer, and Roche, has received grants from Amgen, Boehringer, Bristol-Myers Squibb, Lilly, Merck Sharp & Dohme, Mundipharma, and Roche, and has received non-financial support from Boehringer. CSB’s institution received a grant for this study from Novartis and CSB has received personal fees from Novartis. FB has received personal fees from Novartis. SN has received personal fees from Boehringer, Bristol-Myers Squibb, Eli Lilly, Merck Sharp & Dohme, and Roche. AU, PZ, AD’A, and BM are currently employees of Novartis. AD’A and BM were employees of GlaxoSmithKline during a portion of the study. AD’A and BM own stock in GlaxoSmithKline and Novartis and BM owns stock in Incyte and AstraZeneca. BEJ has received personal fees from Amgen, AstraZeneca, Boehringer, Chugai Pharmaceuticals, Clovis, Genentech, KEW Group, Merck, and Novartis, and shares of post-market revenue for an EGFR genotyping patent. The other authors declare no competing interests.
Figures

Comment in
-
Combined BRAF and MEK inhibition in BRAF-mutant NSCLC.Lancet Oncol. 2016 Jul;17(7):860-862. doi: 10.1016/S1470-2045(16)30203-0. Epub 2016 Jun 6. Lancet Oncol. 2016. PMID: 27283865 No abstract available.
-
Dual inhibition of BRAF and MEK in BRAF-mutated metastatic non-small cell lung cancer.J Thorac Dis. 2016 Sep;8(9):2369-2371. doi: 10.21037/jtd.2016.09.16. J Thorac Dis. 2016. PMID: 27746978 Free PMC article. No abstract available.
Similar articles
-
Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial.Lancet Oncol. 2017 Oct;18(10):1307-1316. doi: 10.1016/S1470-2045(17)30679-4. Epub 2017 Sep 11. Lancet Oncol. 2017. PMID: 28919011 Clinical Trial.
-
Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): a phase 2, open-label, single-arm, multicentre basket trial.Lancet Oncol. 2020 Sep;21(9):1234-1243. doi: 10.1016/S1470-2045(20)30321-1. Epub 2020 Aug 17. Lancet Oncol. 2020. PMID: 32818466 Clinical Trial.
-
Combination of dabrafenib plus trametinib for BRAF and MEK inhibitor pretreated patients with advanced BRAFV600-mutant melanoma: an open-label, single arm, dual-centre, phase 2 clinical trial.Lancet Oncol. 2017 Apr;18(4):464-472. doi: 10.1016/S1470-2045(17)30171-7. Epub 2017 Mar 4. Lancet Oncol. 2017. PMID: 28268064
-
Dabrafenib in combination with trametinib in the treatment of patients with BRAF V600-positive advanced or metastatic non-small cell lung cancer: clinical evidence and experience.Ther Adv Respir Dis. 2018 Jan-Dec;12:1753466618767611. doi: 10.1177/1753466618767611. Ther Adv Respir Dis. 2018. PMID: 29595366 Free PMC article. Review.
-
Dabrafenib and trametinib for the treatment of non-small cell lung cancer.Expert Rev Anticancer Ther. 2018 Nov;18(11):1063-1068. doi: 10.1080/14737140.2018.1521272. Epub 2018 Sep 13. Expert Rev Anticancer Ther. 2018. PMID: 30198802 Review.
Cited by
-
Dabrafenib plus trametinib in patients with relapsed/refractory BRAF V600E mutation-positive hairy cell leukemia.Blood. 2023 Mar 2;141(9):996-1006. doi: 10.1182/blood.2021013658. Blood. 2023. PMID: 36108341 Free PMC article.
-
Promising Response to Dabrafenib Plus Trametinib in a Patient with Peritoneal Carcinomatosis from Non Small Lung Cancer Harboring BRAF V600E Mutation.Onco Targets Ther. 2022 Nov 11;15:1369-1374. doi: 10.2147/OTT.S375246. eCollection 2022. Onco Targets Ther. 2022. PMID: 36388158 Free PMC article.
-
Prognostic Value of Circulating Tumor DNA (ctDNA) in Oncogene-Driven NSCLC: Current Knowledge and Future Perspectives.Cancers (Basel). 2022 Oct 10;14(19):4954. doi: 10.3390/cancers14194954. Cancers (Basel). 2022. PMID: 36230877 Free PMC article. Review.
-
Safety of BRAF+MEK Inhibitor Combinations: Severe Adverse Event Evaluation.Cancers (Basel). 2020 Jun 22;12(6):1650. doi: 10.3390/cancers12061650. Cancers (Basel). 2020. PMID: 32580351 Free PMC article.
-
Prognostic factors in nonsmall cell lung cancer: insights from the German CRISP registry.Eur Respir J. 2023 Feb 2;61(2):2201336. doi: 10.1183/13993003.01336-2022. Print 2023 Feb. Eur Respir J. 2023. PMID: 36180086 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. - PubMed
-
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. - PubMed
-
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–94. - PubMed
-
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. - PubMed
-
- Barlesi F, Mazieres J, Merlio JP, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT) Lancet. 2016;387:1415–26. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

