Coinhibitory Pathways in the B7-CD28 Ligand-Receptor Family
- PMID: 27192563
- PMCID: PMC4905708
- DOI: 10.1016/j.immuni.2016.05.002
Coinhibitory Pathways in the B7-CD28 Ligand-Receptor Family
Abstract
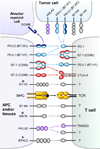
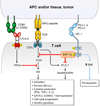
Immune responses need to be controlled for optimal protective immunity and tolerance. Coinhibitory pathways in the B7-CD28 family provide critical inhibitory signals that regulate immune homeostasis and defense and protect tissue integrity. These coinhibitory signals limit the strength and duration of immune responses, thereby curbing immune-mediated tissue damage, regulating resolution of inflammation, and maintaining tolerance to prevent autoimmunity. Tumors and microbes that cause chronic infections can exploit these coinhibitory pathways to establish an immunosuppressive microenvironment, hindering their eradication. Advances in understanding T cell coinhibitory pathways have stimulated a new era of immunotherapy with effective drugs to treat cancer, autoimmune and infectious diseases, and transplant rejection. In this review we discuss the current knowledge of the mechanisms underlying the coinhibitory functions of pathways in the B7-CD28 family, the diverse functional consequences of these inhibitory signals on immune responses, and the overlapping and unique functions of these key immunoregulatory pathways.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Coinhibitory Pathways in Immunotherapy for Cancer.Annu Rev Immunol. 2016 May 20;34:539-73. doi: 10.1146/annurev-immunol-032414-112049. Epub 2016 Feb 25. Annu Rev Immunol. 2016. PMID: 26927206 Review.
-
The B7:CD28 family and friends: Unraveling coinhibitory interactions.Immunity. 2024 Feb 13;57(2):223-244. doi: 10.1016/j.immuni.2024.01.013. Immunity. 2024. PMID: 38354702 Review.
-
Cell intrinsic mechanisms of T-cell inhibition and application to cancer therapy.Immunol Rev. 2008 Aug;224:141-65. doi: 10.1111/j.1600-065X.2008.00649.x. Immunol Rev. 2008. PMID: 18759925 Review.
-
The third group of the B7-CD28 immune checkpoint family: HHLA2, TMIGD2, B7x, and B7-H3.Immunol Rev. 2017 Mar;276(1):26-39. doi: 10.1111/imr.12521. Immunol Rev. 2017. PMID: 28258693 Free PMC article. Review.
-
The CD28-B7 Family of Co-signaling Molecules.Adv Exp Med Biol. 2019;1189:25-51. doi: 10.1007/978-981-32-9717-3_2. Adv Exp Med Biol. 2019. PMID: 31758530 Review.
Cited by
-
The Road Ahead in Pancreatic Cancer: Emerging Trends and Therapeutic Prospects.Biomedicines. 2024 Sep 2;12(9):1979. doi: 10.3390/biomedicines12091979. Biomedicines. 2024. PMID: 39335494 Free PMC article. Review.
-
Exploring the Role of PD-1 in the Autoimmune Response: Insights into Its Implication in Systemic Lupus Erythematosus.Int J Mol Sci. 2024 Jul 15;25(14):7726. doi: 10.3390/ijms25147726. Int J Mol Sci. 2024. PMID: 39062968 Free PMC article. Review.
-
Persistence of lung structural and functional alterations at one year post-COVID-19 is associated with increased serum PD-L2 levels and altered CD4/CD8 ratio.Immun Inflamm Dis. 2024 Jul;12(7):e1305. doi: 10.1002/iid3.1305. Immun Inflamm Dis. 2024. PMID: 39031504 Free PMC article.
-
FAT10 induces immune suppression by upregulating PD-L1 expression in hepatocellular carcinoma.Apoptosis. 2024 Oct;29(9-10):1529-1545. doi: 10.1007/s10495-024-01982-1. Epub 2024 Jun 2. Apoptosis. 2024. PMID: 38824477
-
Prognostic Value of B7H4 Expression in Patients with Solid Cancers: A Systematic Review and Meta-Analysis.Int J Mol Sci. 2024 May 6;25(9):5045. doi: 10.3390/ijms25095045. Int J Mol Sci. 2024. PMID: 38732263 Free PMC article.
References
-
- Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. International immunology. 1996;8:765–772. - PubMed
-
- Alegre ML, Noel PJ, Eisfelder BJ, Chuang E, Clark MR, Reiner SL, Thompson CB. Regulation of surface and intracellular expression of CTLA4 on mouse T cells. J Immunol. 1996;157:4762–4770. - PubMed
-
- Anjos S, Nguyen A, Ounissi-Benkalha H, Tessier MC, Polychronakos C. A common autoimmunity predisposing signal peptide variant of the cytotoxic T-lymphocyte antigen 4 results in inefficient glycosylation of the susceptibility allele. J Biol Chem. 2002;277:46478–46486. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

