CD163-Macrophages Are Involved in Rhabdomyolysis-Induced Kidney Injury and May Be Detected by MRI with Targeted Gold-Coated Iron Oxide Nanoparticles
- PMID: 27162559
- PMCID: PMC4860897
- DOI: 10.7150/thno.14915
CD163-Macrophages Are Involved in Rhabdomyolysis-Induced Kidney Injury and May Be Detected by MRI with Targeted Gold-Coated Iron Oxide Nanoparticles
Abstract
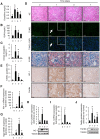
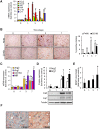
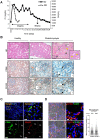
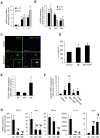
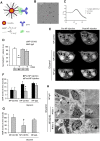
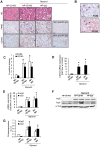
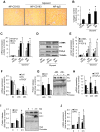
Macrophages play an important role in rhabdomyolysis-acute kidney injury (AKI), although the molecular mechanisms involved in macrophage differentiation are poorly understood. We analyzed the expression and regulation of CD163, a membrane receptor mainly expressed by anti-inflammatory M2 macrophages, in rhabdomyolysis-AKI and developed targeted probes for its specific detection in vivo by MRI. Intramuscular injection of glycerol in mice promoted an early inflammatory response, with elevated proportion of M1 macrophages, and partial differentiation towards a M2 phenotype in later stages, where increased CD163 expression was observed. Immunohistological studies confirmed the presence of CD163-macrophages in human rhabdomyolysis-AKI. In cultured macrophages, myoglobin upregulated CD163 expression via HO-1/IL-10 axis. Moreover, we developed gold-coated iron oxide nanoparticles vectorized with an anti-CD163 antibody that specifically targeted CD163 in kidneys from glycerol-injected mice, as determined by MRI studies, and confirmed by electron microscopy and immunological analysis. Our findings are the first to demonstrate that CD163 is present in both human and experimental rhabdomyolysis-induced AKI, suggesting an important role of this molecule in this pathological condition. Therefore, the use of probes targeting CD163-macrophages by MRI may provide important information about the cellular composition of renal lesion in rhabdomyolysis.
Keywords: CD163; MRI; acute kidney injury.; gold coated iron oxide nanoparticles; macrophages; rhabdomyolysis.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
Targeted gold-coated iron oxide nanoparticles for CD163 detection in atherosclerosis by MRI.Sci Rep. 2015 Nov 30;5:17135. doi: 10.1038/srep17135. Sci Rep. 2015. PMID: 26616677 Free PMC article.
-
Oxidative stress, macrophage infiltration and CD163 expression are determinants of long-term renal outcome in macrohematuria-induced acute kidney injury of IgA nephropathy.Nephron Clin Pract. 2012;121(1-2):c42-53. doi: 10.1159/000342385. Epub 2012 Oct 19. Nephron Clin Pract. 2012. PMID: 23095372
-
Mesenchymal stem cells ameliorate rhabdomyolysis-induced acute kidney injury via the activation of M2 macrophages.Stem Cell Res Ther. 2014 Jun 24;5(3):80. doi: 10.1186/scrt469. Stem Cell Res Ther. 2014. PMID: 24961539 Free PMC article.
-
Phenotypic and functional heterogeneity of the testicular macrophage population: a new regulatory model.J Reprod Immunol. 2013 Apr;97(2):147-58. doi: 10.1016/j.jri.2013.01.001. Epub 2013 Feb 13. J Reprod Immunol. 2013. PMID: 23415010 Review.
-
Expression of macrophage antigens by tumor cells.Adv Exp Med Biol. 2011;714:141-50. doi: 10.1007/978-94-007-0782-5_7. Adv Exp Med Biol. 2011. PMID: 21506012 Review.
Cited by
-
Reduced Chronic Toxicity and Carcinogenicity in A/J Mice in Response to Life-Time Exposure to Aerosol From a Heated Tobacco Product Compared With Cigarette Smoke.Toxicol Sci. 2020 Nov 1;178(1):44-70. doi: 10.1093/toxsci/kfaa131. Toxicol Sci. 2020. PMID: 32780830 Free PMC article.
-
Emerging Strategies of Cancer Therapy Based on Ferroptosis.Adv Mater. 2018 Mar;30(12):e1704007. doi: 10.1002/adma.201704007. Epub 2018 Jan 22. Adv Mater. 2018. PMID: 29356212 Free PMC article. Review.
-
Mild photothermal/radiation therapy potentiates ferroptosis effect for ablation of breast cancer via MRI/PA imaging guided all-in-one strategy.J Nanobiotechnology. 2023 May 8;21(1):150. doi: 10.1186/s12951-023-01910-6. J Nanobiotechnology. 2023. PMID: 37158923 Free PMC article.
-
Role of Macrophages and Related Cytokines in Kidney Disease.Front Med (Lausanne). 2021 Jul 8;8:688060. doi: 10.3389/fmed.2021.688060. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34307414 Free PMC article. Review.
-
Engineered nanodrug targeting oxidative stress for treatment of acute kidney injury.Exploration (Beijing). 2023 Jul 20;3(6):20220148. doi: 10.1002/EXP.20220148. eCollection 2023 Dec. Exploration (Beijing). 2023. PMID: 38264689 Free PMC article. Review.
References
-
- Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361:62–72. - PubMed
-
- Melli G, Chaudhry V, Cornblath DR. Rhabdomyolysis: an evaluation of 475 hospitalized patients. Medicine (Baltimore) 2005;84:377–85. - PubMed
-
- Bagley WH, Yang H, Shah KH. Rhabdomyolysis. Intern Emerg Med. 2007;2:210–8. - PubMed
-
- Kim JH, Lee SS, Jung MH, Yeo HD, Kim HJ, Yang JI. et al. N-acetylcysteine attenuates glycerol-induced acute kidney injury by regulating MAPKs and Bcl-2 family proteins. Nephrol Dial Transplant. 2010;25:1435–43. - PubMed
-
- Homsi E, Janino P, de Faria JB. Role of caspases on cell death, inflammation, and cell cycle in glycerol-induced acute renal failure. Kidney Int. 2006;69:1385–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

