Anti-miR delivery strategies to bypass the blood-brain barrier in glioblastoma therapy
- PMID: 27102443
- PMCID: PMC5045404
- DOI: 10.18632/oncotarget.8837
Anti-miR delivery strategies to bypass the blood-brain barrier in glioblastoma therapy
Abstract
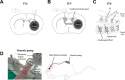
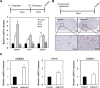
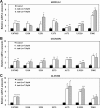
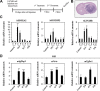
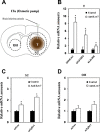
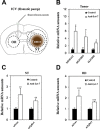
Small non-coding RNAs called miRNAs are key regulators in various biological processes, including tumor initiation, propagation, and metastasis in glioblastoma as well as other cancers. Recent studies have shown the potential for oncogenic miRNAs as therapeutic targets in glioblastoma. However, the application of antisense oligomers, or anti-miRs, to the brain is limited due to the blood-brain barrier (BBB), when administered in the traditional systemic manner. To induce a therapeutic effect in glioblastoma, anti-miR therapy requires a robust and effective delivery system to overcome this obstacle. To bypass the BBB, different delivery administration methods for anti-miRs were evaluated. Stereotaxic surgery was performed to administer anti-Let-7 through intratumoral (ITu), intrathecal (ITh), and intraventricular (ICV) routes, and each method's efficacy was determined by changes in the expression of anti-Let-7 target genes as well as by immunohistochemical analysis. ITu administration of anti-miRs led to a high rate of anti-miR delivery to tumors in the brain by both bolus and continuous administration. In addition, ICV administration, compared with ITu administration, showed a greater distribution of the miR across entire brain tissues. This study suggests that local administration methods are a promising strategy for anti-miR treatment and may overcome current limitations in the treatment of glioblastoma in preclinical animal models.
Keywords: anti-miR; delivery efficiency; glioblastoma; intratumoral injection; intraventricular injection.
Conflict of interest statement
None.
Figures
Similar articles
-
Convection-enhanced delivery of an anti-miR is well-tolerated, preserves anti-miR stability and causes efficient target de-repression: a proof of concept.J Neurooncol. 2016 Jan;126(1):47-55. doi: 10.1007/s11060-015-1947-2. Epub 2015 Oct 1. J Neurooncol. 2016. PMID: 26428358
-
Temporary blood-brain barrier disruption by low intensity pulsed ultrasound increases carboplatin delivery and efficacy in preclinical models of glioblastoma.J Neurooncol. 2019 Aug;144(1):33-41. doi: 10.1007/s11060-019-03204-0. Epub 2019 Jun 13. J Neurooncol. 2019. PMID: 31197598
-
Intratumoral delivery of bortezomib: impact on survival in an intracranial glioma tumor model.J Neurosurg. 2018 Mar;128(3):695-700. doi: 10.3171/2016.11.JNS161212. Epub 2017 Apr 14. J Neurosurg. 2018. PMID: 28409734
-
A comprehensive review in improving delivery of small-molecule chemotherapeutic agents overcoming the blood-brain/brain tumor barriers for glioblastoma treatment.Drug Deliv. 2019 Dec;26(1):551-565. doi: 10.1080/10717544.2019.1616235. Drug Deliv. 2019. PMID: 31928355 Free PMC article. Review.
-
Blood-brain barrier, cytotoxic chemotherapies and glioblastoma.Expert Rev Neurother. 2016 Nov;16(11):1285-1300. doi: 10.1080/14737175.2016.1202761. Epub 2016 Jul 4. Expert Rev Neurother. 2016. PMID: 27310463 Review.
Cited by
-
Targeting Akt-associated microRNAs for cancer therapeutics.Biochem Pharmacol. 2021 Jul;189:114384. doi: 10.1016/j.bcp.2020.114384. Epub 2020 Dec 24. Biochem Pharmacol. 2021. PMID: 33347867 Free PMC article. Review.
-
Drug Delivery Systems in the Development of Novel Strategies for Glioblastoma Treatment.Pharmaceutics. 2022 Jun 1;14(6):1189. doi: 10.3390/pharmaceutics14061189. Pharmaceutics. 2022. PMID: 35745762 Free PMC article. Review.
-
Targeting Non-coding RNA for Glioblastoma Therapy: The Challenge of Overcomes the Blood-Brain Barrier.Front Med Technol. 2021 Aug 10;3:678593. doi: 10.3389/fmedt.2021.678593. eCollection 2021. Front Med Technol. 2021. PMID: 35047931 Free PMC article. Review.
-
Current Perspective on the Natural Compounds and Drug Delivery Techniques in Glioblastoma Multiforme.Cancers (Basel). 2021 Jun 2;13(11):2765. doi: 10.3390/cancers13112765. Cancers (Basel). 2021. PMID: 34199460 Free PMC article. Review.
-
Evolving Landscape of Long Non-coding RNAs in Cerebrospinal Fluid: A Key Role From Diagnosis to Therapy in Brain Tumors.Front Cell Dev Biol. 2021 Oct 7;9:737670. doi: 10.3389/fcell.2021.737670. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34692695 Free PMC article. Review.
References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- van Tellingen O, Yetkin-Arik B, de Gooijer MC, Wesseling P, Wurdinger T, de Vries HE. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist Updat. 2015;19:1–12. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

