cGAS Senses Human Cytomegalovirus and Induces Type I Interferon Responses in Human Monocyte-Derived Cells
- PMID: 27058035
- PMCID: PMC4825940
- DOI: 10.1371/journal.ppat.1005546
cGAS Senses Human Cytomegalovirus and Induces Type I Interferon Responses in Human Monocyte-Derived Cells
Abstract
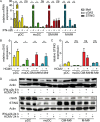
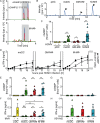
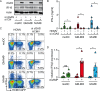
Human cytomegalovirus (HCMV) infections of healthy individuals are mostly unnoticed and result in viral latency. However, HCMV can also cause devastating disease, e.g., upon reactivation in immunocompromised patients. Yet, little is known about human immune cell sensing of DNA-encoded HCMV. Recent studies indicated that during viral infection the cyclic GMP/AMP synthase (cGAS) senses cytosolic DNA and catalyzes formation of the cyclic di-nucleotide cGAMP, which triggers stimulator of interferon genes (STING) and thus induces antiviral type I interferon (IFN-I) responses. We found that plasmacytoid dendritic cells (pDC) as well as monocyte-derived DC and macrophages constitutively expressed cGAS and STING. HCMV infection further induced cGAS, whereas STING expression was only moderately affected. Although pDC expressed particularly high levels of cGAS, and the cGAS/STING axis was functional down-stream of STING, as indicated by IFN-I induction upon synthetic cGAMP treatment, pDC were not susceptible to HCMV infection and mounted IFN-I responses in a TLR9-dependent manner. Conversely, HCMV infected monocyte-derived cells synthesized abundant cGAMP levels that preceded IFN-I production and that correlated with the extent of infection. CRISPR/Cas9- or siRNA-mediated cGAS ablation in monocytic THP-1 cells and primary monocyte-derived cells, respectively, impeded induction of IFN-I responses following HCMV infection. Thus, cGAS is a key sensor of HCMV for IFN-I induction in primary human monocyte-derived DC and macrophages.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Human Cytomegalovirus Tegument Protein pp65 (pUL83) Dampens Type I Interferon Production by Inactivating the DNA Sensor cGAS without Affecting STING.J Virol. 2018 Feb 26;92(6):e01774-17. doi: 10.1128/JVI.01774-17. Print 2018 Mar 15. J Virol. 2018. PMID: 29263269 Free PMC article.
-
cGAMP Quantification in Virus-Infected Human Monocyte-Derived Cells by HPLC-Coupled Tandem Mass Spectrometry.Methods Mol Biol. 2017;1656:153-166. doi: 10.1007/978-1-4939-7237-1_9. Methods Mol Biol. 2017. PMID: 28808968
-
Restriction of Human Cytomegalovirus Replication by ISG15, a Host Effector Regulated by cGAS-STING Double-Stranded-DNA Sensing.J Virol. 2017 Apr 13;91(9):e02483-16. doi: 10.1128/JVI.02483-16. Print 2017 May 1. J Virol. 2017. PMID: 28202760 Free PMC article.
-
cGAS/cGAMP/STING signal propagation in the tumor microenvironment: Key role for myeloid cells in antitumor immunity.Radiother Oncol. 2022 Sep;174:158-167. doi: 10.1016/j.radonc.2022.07.014. Epub 2022 Jul 20. Radiother Oncol. 2022. PMID: 35870728 Review.
-
Intrinsic strategies for the evasion of cGAS-STING signaling-mediated immune surveillance in human cancer: How therapy can overcome them.Pharmacol Res. 2021 Apr;166:105514. doi: 10.1016/j.phrs.2021.105514. Epub 2021 Feb 23. Pharmacol Res. 2021. PMID: 33631336 Review.
Cited by
-
Effect of Cytomegalovirus on the Immune System: Implications for Aging and Mental Health.Curr Top Behav Neurosci. 2023;61:181-214. doi: 10.1007/7854_2022_376. Curr Top Behav Neurosci. 2023. PMID: 35871707
-
Cytosolic DNA Sensing Promotes Macrophage Transformation and Governs Myocardial Ischemic Injury.Circulation. 2018 Jun 12;137(24):2613-2634. doi: 10.1161/CIRCULATIONAHA.117.031046. Epub 2018 Feb 1. Circulation. 2018. PMID: 29437120 Free PMC article.
-
STING induces early IFN-β in the liver and constrains myeloid cell-mediated dissemination of murine cytomegalovirus.Nat Commun. 2019 Jun 27;10(1):2830. doi: 10.1038/s41467-019-10863-0. Nat Commun. 2019. PMID: 31249303 Free PMC article.
-
Cytosolic DNA Sensors and CNS Responses to Viral Pathogens.Front Cell Infect Microbiol. 2020 Sep 16;10:576263. doi: 10.3389/fcimb.2020.576263. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33042875 Free PMC article. Review.
-
IFI44 is an immune evasion biomarker for SARS-CoV-2 and Staphylococcus aureus infection in patients with RA.Front Immunol. 2022 Sep 15;13:1013322. doi: 10.3389/fimmu.2022.1013322. eCollection 2022. Front Immunol. 2022. PMID: 36189314 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

