Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys
- PMID: 26934220
- PMCID: PMC5551389
- DOI: 10.1038/nature17180
Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys
Erratum in
-
Correction to Spotlight Article Published in Vol 11 Issue 4.ACS Chem Biol. 2016 May 20;11(5):1463. doi: 10.1021/acschembio.6b00374. Epub 2016 May 6. ACS Chem Biol. 2016. PMID: 27152473 No abstract available.
Abstract
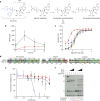
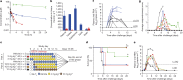
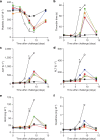
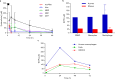
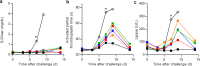
The most recent Ebola virus outbreak in West Africa, which was unprecedented in the number of cases and fatalities, geographic distribution, and number of nations affected, highlights the need for safe, effective, and readily available antiviral agents for treatment and prevention of acute Ebola virus (EBOV) disease (EVD) or sequelae. No antiviral therapeutics have yet received regulatory approval or demonstrated clinical efficacy. Here we report the discovery of a novel small molecule GS-5734, a monophosphoramidate prodrug of an adenosine analogue, with antiviral activity against EBOV. GS-5734 exhibits antiviral activity against multiple variants of EBOV and other filoviruses in cell-based assays. The pharmacologically active nucleoside triphosphate (NTP) is efficiently formed in multiple human cell types incubated with GS-5734 in vitro, and the NTP acts as an alternative substrate and RNA-chain terminator in primer-extension assays using a surrogate respiratory syncytial virus RNA polymerase. Intravenous administration of GS-5734 to nonhuman primates resulted in persistent NTP levels in peripheral blood mononuclear cells (half-life, 14 h) and distribution to sanctuary sites for viral replication including testes, eyes, and brain. In a rhesus monkey model of EVD, once-daily intravenous administration of 10 mg kg(-1) GS-5734 for 12 days resulted in profound suppression of EBOV replication and protected 100% of EBOV-infected animals against lethal disease, ameliorating clinical disease signs and pathophysiological markers, even when treatments were initiated three days after virus exposure when systemic viral RNA was detected in two out of six treated animals. These results show the first substantive post-exposure protection by a small-molecule antiviral compound against EBOV in nonhuman primates. The broad-spectrum antiviral activity of GS-5734 in vitro against other pathogenic RNA viruses, including filoviruses, arenaviruses, and coronaviruses, suggests the potential for wider medical use. GS-5734 is amenable to large-scale manufacturing, and clinical studies investigating the drug safety and pharmacokinetics are ongoing.
Conflict of interest statement
The authors affiliated with Gilead Sciences are employees of the company and may own company stock.
Figures
Similar articles
-
Mechanism of Inhibition of Ebola Virus RNA-Dependent RNA Polymerase by Remdesivir.Viruses. 2019 Apr 4;11(4):326. doi: 10.3390/v11040326. Viruses. 2019. PMID: 30987343 Free PMC article.
-
Will There Be a Cure for Ebola?Annu Rev Pharmacol Toxicol. 2017 Jan 6;57:329-348. doi: 10.1146/annurev-pharmtox-010716-105055. Epub 2016 Dec 7. Annu Rev Pharmacol Toxicol. 2017. PMID: 27959624 Review.
-
Oral administration of obeldesivir protects nonhuman primates against Sudan ebolavirus.Science. 2024 Mar 15;383(6688):eadk6176. doi: 10.1126/science.adk6176. Epub 2024 Mar 15. Science. 2024. PMID: 38484056
-
A single phosphorodiamidate morpholino oligomer targeting VP24 protects rhesus monkeys against lethal Ebola virus infection.mBio. 2015 Feb 10;6(1):e02344-14. doi: 10.1128/mBio.02344-14. mBio. 2015. PMID: 25670780 Free PMC article.
-
Review: Insights on Current FDA-Approved Monoclonal Antibodies Against Ebola Virus Infection.Front Immunol. 2021 Aug 30;12:721328. doi: 10.3389/fimmu.2021.721328. eCollection 2021. Front Immunol. 2021. PMID: 34526994 Free PMC article. Review.
Cited by
-
PREVAIL IV: A Randomized, Double-Blind, 2-Phase, Phase 2 Trial of Remdesivir vs Placebo for Reduction of Ebola Virus RNA in the Semen of Male Survivors.Clin Infect Dis. 2021 Nov 16;73(10):1849-1856. doi: 10.1093/cid/ciab215. Clin Infect Dis. 2021. PMID: 33709142 Free PMC article. Clinical Trial.
-
Effectiveness of remdesivir in patients with COVID-19 under mechanical ventilation in an Italian ICU.J Antimicrob Chemother. 2020 Nov 1;75(11):3359-3365. doi: 10.1093/jac/dkaa321. J Antimicrob Chemother. 2020. PMID: 32829390 Free PMC article.
-
Current approaches used in treating COVID-19 from a molecular mechanisms and immune response perspective.Saudi Pharm J. 2020 Nov;28(11):1333-1352. doi: 10.1016/j.jsps.2020.08.024. Epub 2020 Sep 1. Saudi Pharm J. 2020. PMID: 32905015 Free PMC article. Review.
-
Management of COVID-19 in patients with seizures: Mechanisms of action of potential COVID-19 drug treatments and consideration for potential drug-drug interactions with anti-seizure medications.Epilepsy Res. 2021 Aug;174:106675. doi: 10.1016/j.eplepsyres.2021.106675. Epub 2021 May 19. Epilepsy Res. 2021. PMID: 34044300 Free PMC article. Review.
-
Captisol and GS-704277, but Not GS-441524, Are Credible Mediators of Remdesivir's Nephrotoxicity.Antimicrob Agents Chemother. 2020 Nov 17;64(12):e01920-20. doi: 10.1128/AAC.01920-20. Print 2020 Nov 17. Antimicrob Agents Chemother. 2020. PMID: 32988821 Free PMC article. No abstract available.
References
-
- World Health Organization. Ebola Situation Report - 3 February 2016. http://apps.who.int/ebola/current-situation/ebola-situation-report-3-feb... (2016)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

