Cyclin E Associates with the Lipogenic Enzyme ATP-Citrate Lyase to Enable Malignant Growth of Breast Cancer Cells
- PMID: 26928812
- PMCID: PMC4873469
- DOI: 10.1158/0008-5472.CAN-15-1646
Cyclin E Associates with the Lipogenic Enzyme ATP-Citrate Lyase to Enable Malignant Growth of Breast Cancer Cells
Abstract
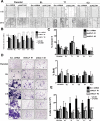
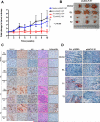
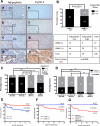
Cyclin E is altered in nearly a third of invasive breast cancers where it is a powerful independent predictor of survival in women with stage I-III disease. Full-length cyclin E is posttranslationally cleaved into low molecular weight (LMW-E) isoforms, which are tumor-specific and accumulate in the cytoplasm because they lack a nuclear localization sequence. We hypothesized that aberrant localization of cytosolic LMW-E isoforms alters target binding and activation ultimately contributing to LMW-E-induced tumorigenicity. To address this hypothesis, we used a retrovirus-based protein complementation assay to find LMW-E binding proteins in breast cancer, identifying ATP-citrate lyase (ACLY), an enzyme in the de novo lipogenesis pathway, as a novel LMW-E-interacting protein in the cytoplasm. LMW-E upregulated ACLY enzymatic activity, subsequently increasing lipid droplet formation, thereby providing cells with essential building blocks to support growth. ACLY was also required for LMW-E-mediated transformation, migration, and invasion of breast cancer cells in vitro along with tumor growth in vivo In clinical specimens of breast cancer, the absence of LMW-E and low expression of adipophilin (PLIN2), a marker of lipid droplet formation, associated with favorable prognosis, whereas overexpression of both proteins correlated with a markedly worse prognosis. Taken together, our findings establish a novel relationship between LMW-E isoforms of cyclin E and aberrant lipid metabolism pathways in breast cancer tumorigenesis, warranting further investigation in additional malignancies exhibiting their expression. Cancer Res; 76(8); 2406-18. ©2016 AACR.
©2016 American Association for Cancer Research.
Figures
Similar articles
-
LMW-E/CDK2 deregulates acinar morphogenesis, induces tumorigenesis, and associates with the activated b-Raf-ERK1/2-mTOR pathway in breast cancer patients.PLoS Genet. 2012;8(3):e1002538. doi: 10.1371/journal.pgen.1002538. Epub 2012 Mar 29. PLoS Genet. 2012. PMID: 22479189 Free PMC article.
-
Overexpression of Cyclin E and its Low Molecular Weight Isoforms Cooperate with Loss of p53 in Promoting Oncogenic Properties of MCF-7 Breast Cancer Cells.Asian Pac J Cancer Prev. 2015;16(17):7575-82. doi: 10.7314/apjcp.2015.16.17.7575. Asian Pac J Cancer Prev. 2015. PMID: 26625764
-
Altered subcellular localization of tumor-specific cyclin E isoforms affects cyclin-dependent kinase 2 complex formation and proteasomal regulation.Cancer Res. 2009 Apr 1;69(7):2817-25. doi: 10.1158/0008-5472.CAN-08-4182. Epub 2009 Mar 24. Cancer Res. 2009. PMID: 19318554 Free PMC article.
-
Low-Molecular-Weight Cyclin E in Human Cancer: Cellular Consequences and Opportunities for Targeted Therapies.Cancer Res. 2018 Oct 1;78(19):5481-5491. doi: 10.1158/0008-5472.CAN-18-1235. Epub 2018 Sep 7. Cancer Res. 2018. PMID: 30194068 Free PMC article. Review.
-
ATP citrate lyase (ACLY) inhibitors: An anti-cancer strategy at the crossroads of glucose and lipid metabolism.Eur J Med Chem. 2018 Sep 5;157:1276-1291. doi: 10.1016/j.ejmech.2018.09.001. Epub 2018 Sep 1. Eur J Med Chem. 2018. PMID: 30195238 Review.
Cited by
-
Non-Coding RNAs Operate in the Crosstalk Between Cancer Metabolic Reprogramming and Metastasis.Front Oncol. 2020 May 29;10:810. doi: 10.3389/fonc.2020.00810. eCollection 2020. Front Oncol. 2020. PMID: 32547948 Free PMC article. Review.
-
Molecular crosstalk between cancer and neurodegenerative diseases.Cell Mol Life Sci. 2020 Jul;77(14):2659-2680. doi: 10.1007/s00018-019-03428-3. Epub 2019 Dec 28. Cell Mol Life Sci. 2020. PMID: 31884567 Free PMC article. Review.
-
Lipid Droplet-Related PLIN2 in CD68+ Tumor-Associated Macrophage of Oral Squamous Cell Carcinoma: Implications for Cancer Prognosis and Immunotherapy.Front Oncol. 2022 Mar 15;12:824235. doi: 10.3389/fonc.2022.824235. eCollection 2022. Front Oncol. 2022. PMID: 35372038 Free PMC article.
-
Cytoplasmic Cyclin E Mediates Resistance to Aromatase Inhibitors in Breast Cancer.Clin Cancer Res. 2017 Dec 1;23(23):7288-7300. doi: 10.1158/1078-0432.CCR-17-1544. Epub 2017 Sep 25. Clin Cancer Res. 2017. PMID: 28947566 Free PMC article.
-
Inter-organelle cross-talk supports acetyl-coenzyme A homeostasis and lipogenesis under metabolic stress.Sci Adv. 2023 May 3;9(18):eadf0138. doi: 10.1126/sciadv.adf0138. Epub 2023 May 3. Sci Adv. 2023. PMID: 37134162 Free PMC article.
References
-
- Mussman JG, Horn HF, Carroll PE, Okuda M, Tarapore P, Donehower LA, et al. Synergistic induction of centrosome hyperamplification by loss of p53 and cyclin E overexpression. Oncogene. 2000;19:1635–46. - PubMed
-
- Keyomarsi K, Tucker SL, Buchholz TA, Callister M, Ding Y, Hortobagyi GN, et al. Cyclin E and survival in patients with breast cancer. The New England journal of medicine. 2002;347:1566–75. - PubMed
-
- Tokai Y, Maeda S, Yamaguchi J, Uga T, Hayashida N, Taniguchi K, et al. Cyclin E low-molecular-weight isoform as a predictor of breast cancer in Japanese women. Int Surg. 2011;96:245–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

