Stabilization of p21 by mTORC1/4E-BP1 predicts clinical outcome of head and neck cancers
- PMID: 26832959
- PMCID: PMC4740818
- DOI: 10.1038/ncomms10438
Stabilization of p21 by mTORC1/4E-BP1 predicts clinical outcome of head and neck cancers
Abstract
The levels, regulation and prognostic value of p21 in head and neck squamous cell carcinomas (HNSCC) has been puzzling for years. Here, we report a new mechanism of regulation of p21 by the mTORC1/4E-BP1 pathway. We find that non-phosphorylated 4E-BP1 interacts with p21 and induces its degradation. Accordingly, hyper-activation of mTORC1 results in phosphorylation of 4E-BP1 and stabilization of p21. In HNSCC, p21 levels strongly correlate with mTORC1 activity but not with p53 status. Finally, clinical data indicate that HNSCC patients with p21 and phospho-S6-double-positive tumours present a better disease-specific survival. We conclude that over-activation of the mTORC1/4E-BP1/p21 pathway is a frequent and clinically relevant alteration in HNSCC.
Figures

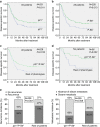
Similar articles
-
AKT inhibition overcomes rapamycin resistance by enhancing the repressive function of PRAS40 on mTORC1/4E-BP1 axis.Oncotarget. 2015 Jun 10;6(16):13962-77. doi: 10.18632/oncotarget.3920. Oncotarget. 2015. PMID: 25961827 Free PMC article.
-
4E-BP1, a multifactor regulated multifunctional protein.Cell Cycle. 2016;15(6):781-6. doi: 10.1080/15384101.2016.1151581. Cell Cycle. 2016. PMID: 26901143 Free PMC article. Review.
-
Overexpressed eIF4E is functionally active in surgical margins of head and neck cancer patients via activation of the Akt/mammalian target of rapamycin pathway.Clin Cancer Res. 2004 Sep 1;10(17):5820-7. doi: 10.1158/1078-0432.CCR-03-0483. Clin Cancer Res. 2004. PMID: 15355912
-
Constitutive phosphorylation of the mTORC2/Akt/4E-BP1 pathway in newly derived canine hemangiosarcoma cell lines.BMC Vet Res. 2012 Jul 29;8:128. doi: 10.1186/1746-6148-8-128. BMC Vet Res. 2012. PMID: 22839755 Free PMC article.
-
Reappraisal to the study of 4E-BP1 as an mTOR substrate - A normative critique.Eur J Cell Biol. 2017 Jun;96(4):325-336. doi: 10.1016/j.ejcb.2017.03.013. Epub 2017 Apr 8. Eur J Cell Biol. 2017. PMID: 28427795 Review.
Cited by
-
miR-125a-5p Functions as Tumor Suppressor microRNA And Is a Marker of Locoregional Recurrence And Poor prognosis in Head And Neck Cancer.Neoplasia. 2019 Sep;21(9):849-862. doi: 10.1016/j.neo.2019.06.004. Epub 2019 Jul 18. Neoplasia. 2019. PMID: 31325708 Free PMC article.
-
Eukaryotic initiation factor 4E-binding protein as an oncogene in breast cancer.BMC Cancer. 2019 May 23;19(1):491. doi: 10.1186/s12885-019-5667-4. BMC Cancer. 2019. PMID: 31122207 Free PMC article.
-
Overcoming adaptive resistance in mucoepidermoid carcinoma through inhibition of the IKK-β/IκBα/NFκB axis.Oncotarget. 2016 Nov 8;7(45):73032-73044. doi: 10.18632/oncotarget.12195. Oncotarget. 2016. PMID: 27682876 Free PMC article.
-
Role of mTOR through Autophagy in Esophageal Cancer Stemness.Cancers (Basel). 2022 Apr 1;14(7):1806. doi: 10.3390/cancers14071806. Cancers (Basel). 2022. PMID: 35406578 Free PMC article.
-
Characterization of Molecular Subtypes in Head and Neck Squamous Cell Carcinoma With Distinct Prognosis and Treatment Responsiveness.Front Cell Dev Biol. 2021 Sep 14;9:711348. doi: 10.3389/fcell.2021.711348. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34595167 Free PMC article.
References
-
- Warfel N. A. & El-Deiry W. S. p21WAF1 and tumourigenesis: 20 years after. Curr. Opin. Oncol. 25, 52–58 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

