The RNA-binding protein Mex3B is a coreceptor of Toll-like receptor 3 in innate antiviral response
- PMID: 26823206
- PMCID: PMC4783467
- DOI: 10.1038/cr.2016.16
The RNA-binding protein Mex3B is a coreceptor of Toll-like receptor 3 in innate antiviral response
Abstract
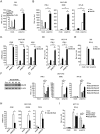
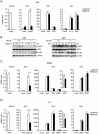
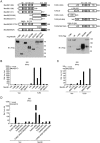
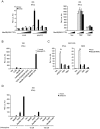
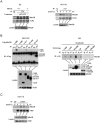
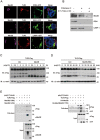
Recognition of viral dsRNA by Toll-like receptor 3 (TLR3) leads to induction of interferons (IFNs) and proinflammatory cytokines, and innate antiviral response. Here we identified the RNA-binding protein Mex3B as a positive regulator of TLR3-mediated signaling by expression cloning screens. Cells from Mex3b(-/-) mice exhibited reduced production of IFN-β in response to the dsRNA analog poly(I:C) but not infection with RNA viruses. Mex3b(-/-) mice injected with poly(I:C) was more resistant to poly(I:C)-induced death. Mex3B was associated with TLR3 in the endosomes. It bound to dsRNA and increased the dsRNA-binding activity of TLR3. Mex3B also promoted the proteolytic processing of TLR3, which is critical for its activation. Mutants of Mex3B lacking its RNA-binding activity inhibited TLR3-mediated IFN-β induction. These findings suggest that Mex3B acts as a coreceptor of TLR3 in innate antiviral response.
Figures


Comment in
-
Mex3B: a coreceptor to present dsRNA to TLR3.Cell Res. 2016 Apr;26(4):391-2. doi: 10.1038/cr.2016.29. Epub 2016 Mar 4. Cell Res. 2016. PMID: 26940660 Free PMC article.
Similar articles
-
Mex3B: a coreceptor to present dsRNA to TLR3.Cell Res. 2016 Apr;26(4):391-2. doi: 10.1038/cr.2016.29. Epub 2016 Mar 4. Cell Res. 2016. PMID: 26940660 Free PMC article.
-
Interferon β (IFN-β) Production during the Double-stranded RNA (dsRNA) Response in Hepatocytes Involves Coordinated and Feedforward Signaling through Toll-like Receptor 3 (TLR3), RNA-dependent Protein Kinase (PKR), Inducible Nitric Oxide Synthase (iNOS), and Src Protein.J Biol Chem. 2016 Jul 15;291(29):15093-107. doi: 10.1074/jbc.M116.717942. Epub 2016 May 17. J Biol Chem. 2016. PMID: 27226571 Free PMC article.
-
Interferon-beta induction through toll-like receptor 3 depends on double-stranded RNA structure.DNA Cell Biol. 2005 Oct;24(10):614-23. doi: 10.1089/dna.2005.24.614. DNA Cell Biol. 2005. PMID: 16225392
-
Functional evolution of the TICAM-1 pathway for extrinsic RNA sensing.Immunol Rev. 2009 Jan;227(1):44-53. doi: 10.1111/j.1600-065X.2008.00723.x. Immunol Rev. 2009. PMID: 19120474 Review.
-
Antiviral responses induced by the TLR3 pathway.Rev Med Virol. 2011 Mar;21(2):67-77. doi: 10.1002/rmv.680. Epub 2011 Feb 10. Rev Med Virol. 2011. PMID: 21312311 Review.
Cited by
-
Activation of the STAT3 Signaling Pathway by the RNA-Dependent RNA Polymerase Protein of Arenavirus.Viruses. 2021 May 25;13(6):976. doi: 10.3390/v13060976. Viruses. 2021. PMID: 34070281 Free PMC article.
-
Low expression of RNA sensors impacts Zika virus infection in the lower female reproductive tract.Nat Commun. 2019 Sep 25;10(1):4344. doi: 10.1038/s41467-019-12371-7. Nat Commun. 2019. PMID: 31554802 Free PMC article.
-
Human Cytomegalovirus Protein UL94 Targets MITA to Evade the Antiviral Immune Response.J Virol. 2020 Jun 1;94(12):e00022-20. doi: 10.1128/JVI.00022-20. Print 2020 Jun 1. J Virol. 2020. PMID: 32238587 Free PMC article.
-
Ubiquitination of TLR3 by TRIM3 signals its ESCRT-mediated trafficking to the endolysosomes for innate antiviral response.Proc Natl Acad Sci U S A. 2020 Sep 22;117(38):23707-23716. doi: 10.1073/pnas.2002472117. Epub 2020 Sep 2. Proc Natl Acad Sci U S A. 2020. PMID: 32878999 Free PMC article.
-
Investigation of the Potential Correlation Between RNA-Binding Proteins in the Evolutionarily Conserved MEX3 Family and Non-small-Cell Lung Cancer.Mol Biotechnol. 2023 Aug;65(8):1263-1274. doi: 10.1007/s12033-022-00638-2. Epub 2022 Dec 12. Mol Biotechnol. 2023. PMID: 36507941 Free PMC article.
References
-
- Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011; 34:637–650. - PubMed
-
- Jin MS, Lee JO. Structures of the toll-like receptor family and its ligand complexes. Immunity 2008; 29:182–191. - PubMed
-
- Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity 2010; 32:305–315. - PubMed
-
- McGettrick AF, O'Neill LA. Localisation and trafficking of Toll-like receptors: an important mode of regulation. Curr Opin Immunol 2010; 22:20–27. - PubMed
-
- Matsumoto M, Funami K, Tanabe M, et al. Subcellular localization of Toll-like receptor 3 in human dendritic cells. J Immunol 2003; 171:3154–3162. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

