Human Mesenchymal Stem Cells of Diverse Origins Support Persistent Infection with Kaposi's Sarcoma-Associated Herpesvirus and Manifest Distinct Angiogenic, Invasive, and Transforming Phenotypes
- PMID: 26814175
- PMCID: PMC4742711
- DOI: 10.1128/mBio.02109-15
Human Mesenchymal Stem Cells of Diverse Origins Support Persistent Infection with Kaposi's Sarcoma-Associated Herpesvirus and Manifest Distinct Angiogenic, Invasive, and Transforming Phenotypes
Abstract
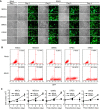
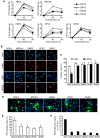
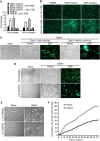
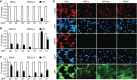
Kaposi's sarcoma (KS), a highly angiogenic and invasive tumor often involving different organ sites, including the oral cavity, is caused by infection with Kaposi's sarcoma-associated herpesvirus (KSHV). Diverse cell markers have been identified on KS tumor cells, but their origin remains an enigma. We previously showed that KSHV could efficiently infect, transform, and reprogram rat primary mesenchymal stem cells (MSCs) into KS-like tumor cells. In this study, we showed that human primary MSCs derived from diverse organs, including bone marrow (MSCbm), adipose tissue (MSCa), dental pulp, gingiva tissue (GMSC), and exfoliated deciduous teeth, were permissive to KSHV infection. We successfully established long-term cultures of KSHV-infected MSCa, MSCbm, and GMSC (LTC-KMSCs). While LTC-KMSCs had lower proliferation rates than the uninfected cells, they expressed mixtures of KS markers and displayed differential angiogenic, invasive, and transforming phenotypes. Genetic analysis identified KSHV-derived microRNAs that mediated KSHV-induced angiogenic activity by activating the AKT pathway. These results indicated that human MSCs could be the KSHV target cells in vivo and established valid models for delineating the mechanism of KSHV infection, replication, and malignant transformation in biologically relevant cell types.
Importance: Kaposi's sarcoma is the most common cancer in AIDS patients. While KSHV infection is required for the development of Kaposi's sarcoma, the origin of KSHV target cells remains unclear. We show that KSHV can efficiently infect human primary mesenchymal stem cells of diverse origins and reprogram them to acquire various degrees of Kaposi's sarcoma-like cell makers and angiogenic, invasive, and transforming phenotypes. These results indicate that human mesenchymal stem cells might be the KSHV target cells and establish models for delineating the mechanism of KSHV-induced malignant transformation.
Copyright © 2016 Lee et al.
Figures

Similar articles
-
Pseudomonas aeruginosa Stimulates Inflammation and Enhances Kaposi's Sarcoma Herpesvirus-Induced Cell Proliferation and Cellular Transformation through both Lipopolysaccharide and Flagellin.mBio. 2020 Nov 10;11(6):e02843-20. doi: 10.1128/mBio.02843-20. mBio. 2020. PMID: 33173008 Free PMC article.
-
Kaposi's Sarcoma-Associated Herpesvirus Infection Induces the Expression of Neuroendocrine Genes in Endothelial Cells.J Virol. 2020 Mar 31;94(8):e01692-19. doi: 10.1128/JVI.01692-19. Print 2020 Mar 31. J Virol. 2020. PMID: 31969437 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus downregulates transforming growth factor β2 to promote enhanced stability of capillary-like tube formation.J Virol. 2014 Dec;88(24):14301-9. doi: 10.1128/JVI.01696-14. Epub 2014 Oct 1. J Virol. 2014. PMID: 25275137 Free PMC article.
-
KSHV-Mediated Angiogenesis in Tumor Progression.Viruses. 2016 Jul 20;8(7):198. doi: 10.3390/v8070198. Viruses. 2016. PMID: 27447661 Free PMC article. Review.
-
Kaposi's sarcoma herpesvirus-induced endothelial cell reprogramming supports viral persistence and contributes to Kaposi's sarcoma tumorigenesis.Curr Opin Virol. 2017 Oct;26:156-162. doi: 10.1016/j.coviro.2017.09.002. Epub 2017 Oct 12. Curr Opin Virol. 2017. PMID: 29031103 Review.
Cited by
-
HIV-associated cancers and lymphoproliferative disorders caused by Kaposi sarcoma herpesvirus and Epstein-Barr virus.Clin Microbiol Rev. 2024 Sep 12;37(3):e0002223. doi: 10.1128/cmr.00022-23. Epub 2024 Jun 20. Clin Microbiol Rev. 2024. PMID: 38899877 Review.
-
Curcumin is an APE1 redox inhibitor and exhibits an antiviral activity against KSHV replication and pathogenesis.Antiviral Res. 2019 Jul;167:98-103. doi: 10.1016/j.antiviral.2019.04.011. Epub 2019 Apr 26. Antiviral Res. 2019. PMID: 31034848 Free PMC article.
-
Kaposi's sarcoma herpesvirus activates the hypoxia response to usurp HIF2α-dependent translation initiation for replication and oncogenesis.Cell Rep. 2021 Dec 28;37(13):110144. doi: 10.1016/j.celrep.2021.110144. Cell Rep. 2021. PMID: 34965440 Free PMC article.
-
DLX1008 (brolucizumab), a single-chain anti-VEGF-A antibody fragment with low picomolar affinity, leads to tumor involution in an in vivo model of Kaposi Sarcoma.PLoS One. 2020 May 14;15(5):e0233116. doi: 10.1371/journal.pone.0233116. eCollection 2020. PLoS One. 2020. PMID: 32407363 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus at 27.Tumour Virus Res. 2021 Dec;12:200223. doi: 10.1016/j.tvr.2021.200223. Epub 2021 Jun 19. Tumour Virus Res. 2021. PMID: 34153523 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA096512/CA/NCI NIH HHS/United States
- R01 CA124332/CA/NCI NIH HHS/United States
- AI073099/AI/NIAID NIH HHS/United States
- R01 CA082057/CA/NCI NIH HHS/United States
- R01 DE025465/DE/NIDCR NIH HHS/United States
- CA31363/CA/NCI NIH HHS/United States
- R01 AI073099/AI/NIAID NIH HHS/United States
- R01 CA031363/CA/NCI NIH HHS/United States
- DE025465/DE/NIDCR NIH HHS/United States
- R01 CA197153/CA/NCI NIH HHS/United States
- CA132637/CA/NCI NIH HHS/United States
- CA096512/CA/NCI NIH HHS/United States
- CA197153/CA/NCI NIH HHS/United States
- R01 DE023926/DE/NIDCR NIH HHS/United States
- P01 CA180779/CA/NCI NIH HHS/United States
- R01 CA115284/CA/NCI NIH HHS/United States
- R01 CA177377/CA/NCI NIH HHS/United States
- CA082057/CA/NCI NIH HHS/United States
- CA115284/CA/NCI NIH HHS/United States
- AI105809/AI/NIAID NIH HHS/United States
- R01 CA132637/CA/NCI NIH HHS/United States
- R01 CA119917/CA/NCI NIH HHS/United States
- CA124332/CA/NCI NIH HHS/United States
- CA177377/CA/NCI NIH HHS/United States
- CA180779/CA/NCI NIH HHS/United States
- R35 CA200422/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

