Altered Skeletal Muscle Mitochondrial Proteome As the Basis of Disruption of Mitochondrial Function in Diabetic Mice
- PMID: 26718503
- PMCID: PMC4764144
- DOI: 10.2337/db15-0823
Altered Skeletal Muscle Mitochondrial Proteome As the Basis of Disruption of Mitochondrial Function in Diabetic Mice
Abstract
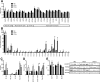
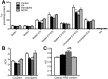
Insulin plays pivotal role in cellular fuel metabolism in skeletal muscle. Despite being the primary site of energy metabolism, the underlying mechanism on how insulin deficiency deranges skeletal muscle mitochondrial physiology remains to be fully understood. Here we report an important link between altered skeletal muscle proteome homeostasis and mitochondrial physiology during insulin deficiency. Deprivation of insulin in streptozotocin-induced diabetic mice decreased mitochondrial ATP production, reduced coupling and phosphorylation efficiency, and increased oxidant emission in skeletal muscle. Proteomic survey revealed that the mitochondrial derangements during insulin deficiency were related to increased mitochondrial protein degradation and decreased protein synthesis, resulting in reduced abundance of proteins involved in mitochondrial respiration and β-oxidation. However, a paradoxical upregulation of proteins involved in cellular uptake of fatty acids triggered an accumulation of incomplete fatty acid oxidation products in skeletal muscle. These data implicate a mismatch of β-oxidation and fatty acid uptake as a mechanism leading to increased oxidative stress in diabetes. This notion was supported by elevated oxidative stress in cultured myotubes exposed to palmitate in the presence of a β-oxidation inhibitor. Together, these results indicate that insulin deficiency alters the balance of proteins involved in fatty acid transport and oxidation in skeletal muscle, leading to impaired mitochondrial function and increased oxidative stress.
© 2016 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
Figures



Similar articles
-
Effect of puerarin in promoting fatty acid oxidation by increasing mitochondrial oxidative capacity and biogenesis in skeletal muscle in diabetic rats.Nutr Diabetes. 2018 Jan 12;8(1):1. doi: 10.1038/s41387-017-0009-6. Nutr Diabetes. 2018. PMID: 29330446 Free PMC article.
-
Remodeling of skeletal muscle mitochondrial proteome with high-fat diet involves greater changes to β-oxidation than electron transfer proteins in mice.Am J Physiol Endocrinol Metab. 2018 Oct 1;315(4):E425-E434. doi: 10.1152/ajpendo.00051.2018. Epub 2018 May 29. Am J Physiol Endocrinol Metab. 2018. PMID: 29812987 Free PMC article.
-
Long-chain acyl-CoA synthetase 6 regulates lipid synthesis and mitochondrial oxidative capacity in human and rat skeletal muscle.J Physiol. 2017 Feb 1;595(3):677-693. doi: 10.1113/JP272962. Epub 2016 Nov 8. J Physiol. 2017. PMID: 27647415 Free PMC article.
-
Altered mitochondrial function in insulin-deficient and insulin-resistant states.J Clin Invest. 2018 Aug 31;128(9):3671-3681. doi: 10.1172/JCI120843. Epub 2018 Aug 31. J Clin Invest. 2018. PMID: 30168804 Free PMC article. Review.
-
Role of fatty acid uptake and fatty acid beta-oxidation in mediating insulin resistance in heart and skeletal muscle.Biochim Biophys Acta. 2010 Jan;1801(1):1-22. doi: 10.1016/j.bbalip.2009.09.014. Epub 2009 Sep 24. Biochim Biophys Acta. 2010. PMID: 19782765 Review.
Cited by
-
The Effect of Glucagon on Protein Catabolism During Insulin Deficiency: Exchange of Amino Acids Across Skeletal Muscle and the Splanchnic Bed.Diabetes. 2022 Aug 1;71(8):1636-1648. doi: 10.2337/db22-0079. Diabetes. 2022. PMID: 35621914 Free PMC article.
-
Loss of FoxOs in muscle reveals sex-based differences in insulin sensitivity but mitigates diet-induced obesity.Mol Metab. 2019 Dec;30:203-220. doi: 10.1016/j.molmet.2019.10.001. Epub 2019 Oct 10. Mol Metab. 2019. PMID: 31767172 Free PMC article.
-
Skeletal muscle ex vivo mitochondrial respiration parallels decline in vivo oxidative capacity, cardiorespiratory fitness, and muscle strength: The Baltimore Longitudinal Study of Aging.Aging Cell. 2018 Apr;17(2):e12725. doi: 10.1111/acel.12725. Epub 2018 Jan 21. Aging Cell. 2018. PMID: 29356348 Free PMC article.
-
Proteomics of Skeletal Muscle: Focus on Insulin Resistance and Exercise Biology.Proteomes. 2016 Feb 4;4(1):6. doi: 10.3390/proteomes4010006. Proteomes. 2016. PMID: 28248217 Free PMC article.
-
Untangling the genetic link between type 1 and type 2 diabetes using functional genomics.Sci Rep. 2021 Jul 6;11(1):13871. doi: 10.1038/s41598-021-93346-x. Sci Rep. 2021. PMID: 34230558 Free PMC article.
References
-
- Boirie Y, Short KR, Ahlman B, Charlton M, Nair KS. Tissue-specific regulation of mitochondrial and cytoplasmic protein synthesis rates by insulin. Diabetes 2001;50:2652–2658 - PubMed
-
- Rizza RA, Jensen MD, Nair KS. Type 1 diabetes mellitus (insulin-dependent diabetes mellitus). In Handbook of Physiology. Jefferson LS, Cherrington AD, Goodman HM, Eds. Oxford University Press, 2001, p. 1093–1114
-
- Karakelides H, Asmann YW, Bigelow ML, et al. . Effect of insulin deprivation on muscle mitochondrial ATP production and gene transcript levels in type 1 diabetic subjects. Diabetes 2007;56:2683–2689 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

