Population pharmacokinetic and exposure-response analysis for trastuzumab administered using a subcutaneous "manual syringe" injection or intravenously in women with HER2-positive early breast cancer
- PMID: 26645407
- PMCID: PMC4706584
- DOI: 10.1007/s00280-015-2922-5
Population pharmacokinetic and exposure-response analysis for trastuzumab administered using a subcutaneous "manual syringe" injection or intravenously in women with HER2-positive early breast cancer
Abstract
Purpose: To characterize the population pharmacokinetics (PKs) of subcutaneous (SC) and intravenous (IV) trastuzumab in early breast cancer (EBC), assess the impact of covariates on trastuzumab PK, and evaluate fixed (nonweight-based) dosing for the SC regimen administrated via handheld syringe.
Methods: Serum trastuzumab concentrations from 595 patients with HER2-positive EBC in the HannaH study (fixed 600 mg SC trastuzumab or weight-based IV trastuzumab) were analyzed using nonlinear mixed-effects modeling. Multiple logistic regression was used to assess the exposure-response relationships between PK, efficacy [pathologic complete response (pCR)], and safety [grade ≥3 adverse events (AEs)].
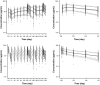
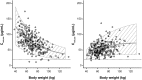
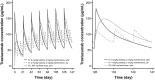
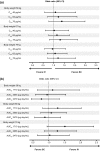
Results: Trastuzumab PK was described by a two-compartment model with parallel linear and nonlinear elimination and first-order SC absorption, with a bioavailability of 77 %. Estimated total clearance (CL) values were 0.18-0.22 L/day for steady-state trough/peak concentrations of 75-148 µg/mL; the estimate for central volume of distribution was 2.9 L. Body weight and alanine transaminase, while showing significant effects on PK, only explained 8% of the variability in CL. Exposure-response analyses showed no relationship between PK, pCR, and grade ≥3 AEs for either regimen.
Conclusion: A fixed 600 mg SC dose of trastuzumab provides the desired exposure, with steady-state trough concentrations (35-123 μg/mL for the 5th-95th percentiles) above the historical target concentration of 20 μg/mL for efficacy. Fixed dosing is further supported by lack of an exposure-response relationship between PK, pCR, and grade ≥3 AEs. No dose adjustment per patient factors is required within the ranges studied.
Keywords: Early breast cancer; Fixed dose; HER2; NONMEM; Population pharmacokinetics modeling; Trastuzumab.
Figures
Similar articles
-
Population pharmacokinetic and exploratory exposure-response analysis of the fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection in patients with HER2-positive early breast cancer in the FeDeriCa study.Cancer Chemother Pharmacol. 2021 Sep;88(3):499-512. doi: 10.1007/s00280-021-04296-0. Epub 2021 Jun 9. Cancer Chemother Pharmacol. 2021. PMID: 34106303 Free PMC article. Clinical Trial.
-
Subcutaneous versus intravenous formulation of trastuzumab for HER2-positive early breast cancer: updated results from the phase III HannaH study.Ann Oncol. 2015 Feb;26(2):320-5. doi: 10.1093/annonc/mdu524. Epub 2014 Nov 17. Ann Oncol. 2015. PMID: 25403587 Clinical Trial.
-
Pharmacokinetic and exposure-response analyses of pertuzumab in combination with trastuzumab and docetaxel during neoadjuvant treatment of HER2+ early breast cancer.Cancer Chemother Pharmacol. 2017 Feb;79(2):353-361. doi: 10.1007/s00280-016-3218-0. Epub 2017 Jan 10. Cancer Chemother Pharmacol. 2017. PMID: 28074265 Free PMC article.
-
Is weight-based IV dosing of trastuzumab preferable to SC fixed-dose in some patients? A systematic scoping review.Breast. 2021 Jun;57:95-103. doi: 10.1016/j.breast.2021.03.003. Epub 2021 Mar 18. Breast. 2021. PMID: 33799233 Free PMC article. Review.
-
Subcutaneous Trastuzumab: A Review in HER2-Positive Breast Cancer.Target Oncol. 2019 Dec;14(6):749-758. doi: 10.1007/s11523-019-00684-y. Target Oncol. 2019. PMID: 31686307 Review.
Cited by
-
Subcutaneous Trastuzumab: An Observational Study of Safety and Tolerability in Patients With Early HER2-Positive Breast Cancer.Int J Breast Cancer. 2024 Jun 22;2024:9551710. doi: 10.1155/2024/9551710. eCollection 2024. Int J Breast Cancer. 2024. PMID: 38962673 Free PMC article.
-
Development of a Subcutaneous Fixed-Dose Combination of Pertuzumab and Trastuzumab: Results From the Phase Ib Dose-Finding Study.J Clin Pharmacol. 2019 May;59(5):702-716. doi: 10.1002/jcph.1362. Epub 2018 Dec 19. J Clin Pharmacol. 2019. PMID: 30570763 Free PMC article. Clinical Trial.
-
Exposure-Response and Population Pharmacokinetic Analyses of a Novel Subcutaneous Formulation of Daratumumab Administered to Multiple Myeloma Patients.J Clin Pharmacol. 2021 May;61(5):614-627. doi: 10.1002/jcph.1771. Epub 2020 Nov 3. J Clin Pharmacol. 2021. PMID: 33145788 Free PMC article.
-
Trastuzumab increases pulmonary vein arrhythmogenesis through modulating pulmonary vein electrical and conduction properties via phosphatidylinositol 3-kinase signaling.Iran J Basic Med Sci. 2020 Jul;23(7):865-870. doi: 10.22038/ijbms.2020.44651.10432. Iran J Basic Med Sci. 2020. PMID: 32774807 Free PMC article.
-
Antibody Drug Clearance: An Underexplored Marker of Outcomes with Checkpoint Inhibitors.Clin Cancer Res. 2024 Mar 1;30(5):942-958. doi: 10.1158/1078-0432.CCR-23-1683. Clin Cancer Res. 2024. PMID: 37921739 Free PMC article. Review.
References
-
- Sliwkowski MX, Lofgren JA, Lewis GD, Hotaling TE, Fendly BM, Fox JA. Nonclinical studies addressing the mechanism of action of trastuzumab (Herceptin) Semin Oncol. 1999;26:60–70. - PubMed
-
- Herceptin: Prescribing Information (2014) http://www.gene.com/download/pdf/herceptin_prescribing.pdf. Accessed 1 Oct 2014
-
- Herceptin: Summary of Product Characteristics (2011) http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Info.... Accessed 1 Oct 2014
-
- Wynne C, Harvey V, Schwabe C, Waaka D, McIntyre C, Bittner B. Comparison of subcutaneous and intravenous administration of trastuzumab: a phase I/Ib trial in healthy male volunteers and patients with HER2-positive breast cancer. J Clin Pharmacol. 2013;53:192–201. doi: 10.1177/0091270012436560. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

