Dinaciclib, a Cyclin-Dependent Kinase Inhibitor Promotes Proteasomal Degradation of Mcl-1 and Enhances ABT-737-Mediated Cell Death in Malignant Human Glioma Cell Lines
- PMID: 26585571
- PMCID: PMC6047232
- DOI: 10.1124/jpet.115.230052
Dinaciclib, a Cyclin-Dependent Kinase Inhibitor Promotes Proteasomal Degradation of Mcl-1 and Enhances ABT-737-Mediated Cell Death in Malignant Human Glioma Cell Lines
Abstract
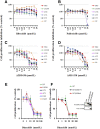
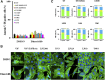
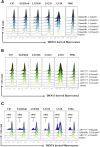
The prognosis for malignant glioma, the most common brain tumor, is still poor, underscoring the need to develop novel treatment strategies. Because glioma cells commonly exhibit genomic alterations involving genes that regulate cell-cycle control, there is a strong rationale for examining the potential efficacy of strategies to counteract this process. In this study, we examined the antiproliferative effects of the cyclin-dependent kinase inhibitor dinaciclib in malignant human glioma cell lines, with intact, deleted, or mutated p53 or phosphatase and tensin homolog on chromosome 10; intact or deleted or p14ARF or wild-type or amplified epidermal growth factor receptor. Dinaciclib inhibited cell proliferation and induced cell-cycle arrest at the G2/M checkpoint, independent of p53 mutational status. In a standard 72-hour 3-[4,5-dimethylthiazol- 2yl]-5-[3-carboxymethoxyphenyl]-2-[4-sulfophenyl]-2H, tetrazolium (MTS) assay, at clinically relevant concentrations, dose-dependent antiproliferative effects were observed, but cell death was not induced. Moreover, the combination of conventional chemotherapeutic agents and various growth-signaling inhibitors with dinaciclib did not yield synergistic cytotoxicity. In contrast, combination of the Bcl-2/Bcl-xL inhibitors ABT-263 (4-[4-[[2-(4-chlorophenyl)-5,5-dimethylcyclohexen-1-yl]methyl]piperazin-1-yl]-N-[4-[[(2R)-4-morpholin-4-yl-1-phenylsulfanylbutan-2-yl]amino]-3-(trifluoromethylsulfonyl)phenyl]sulfonylbenzamide) or ABT-737 (4-[4-[[2-(4-chlorophenyl)phenyl]methyl]piperazin-1-yl]-N-[4-[[(2R)-4-(dimethylamino)-1-phenylsulfanylbutan-2-yl]amino]-3-nitrophenyl]sulfonylbenzamide) with dinaciclib potentiated the apoptotic response induced by each single drug. The synergistic killing by ABT-737 with dinaciclib led to cell death accompanied by the hallmarks of apoptosis, including an early loss of the mitochondrial transmembrane potential; the release of cytochrome c, smac/DIABLO, and apoptosis-inducing factor; phosphatidylserine exposure on the plasma membrane surface and activation of caspases and poly ADP-ribose polymerase. Mechanistic studies revealed that dinaciclib promoted proteasomal degradation of Mcl-1. These observations may have important clinical implications for the design of experimental treatment protocols for malignant human glioma.
Copyright © 2016 by The American Society for Pharmacology and Experimental Therapeutics.
Figures




Similar articles
-
Cucurbitacin-I inhibits Aurora kinase A, Aurora kinase B and survivin, induces defects in cell cycle progression and promotes ABT-737-induced cell death in a caspase-independent manner in malignant human glioma cells.Cancer Biol Ther. 2015;16(2):233-43. doi: 10.4161/15384047.2014.987548. Cancer Biol Ther. 2015. PMID: 25482928 Free PMC article.
-
Mitochondrial dysfunction RAD51, and Ku80 proteolysis promote apoptotic effects of Dinaciclib in Bcl-xL silenced cells.Mol Carcinog. 2018 Apr;57(4):469-482. doi: 10.1002/mc.22771. Epub 2017 Dec 30. Mol Carcinog. 2018. PMID: 29240261 Free PMC article.
-
Cyclin E/Cdk2-dependent phosphorylation of Mcl-1 determines its stability and cellular sensitivity to BH3 mimetics.Oncotarget. 2015 Jul 10;6(19):16912-25. doi: 10.18632/oncotarget.4857. Oncotarget. 2015. PMID: 26219338 Free PMC article.
-
p53-dependent regulation of Mcl-1 contributes to synergistic cell death by ionizing radiation and the Bcl-2/Bcl-XL inhibitor ABT-737.Apoptosis. 2012 Feb;17(2):187-99. doi: 10.1007/s10495-011-0664-3. Apoptosis. 2012. PMID: 22002102
-
Inhibition of phosphatidylinositol 3-kinase/AKT signaling by NVP-BKM120 promotes ABT-737-induced toxicity in a caspase-dependent manner through mitochondrial dysfunction and DNA damage response in established and primary cultured glioblastoma cells.J Pharmacol Exp Ther. 2014 Jul;350(1):22-35. doi: 10.1124/jpet.114.212910. Epub 2014 Apr 16. J Pharmacol Exp Ther. 2014. PMID: 24741074 Free PMC article.
Cited by
-
Development of an MCL-1-related prognostic signature and inhibitors screening for glioblastoma.Front Pharmacol. 2023 Jul 19;14:1162540. doi: 10.3389/fphar.2023.1162540. eCollection 2023. Front Pharmacol. 2023. PMID: 37538176 Free PMC article.
-
DCAF7 regulates cell proliferation through IRS1-FOXO1 signaling.iScience. 2022 Sep 24;25(10):105188. doi: 10.1016/j.isci.2022.105188. eCollection 2022 Oct 21. iScience. 2022. PMID: 36248734 Free PMC article.
-
Co-targeting CDK2 and CDK4/6 overcomes resistance to aromatase and CDK4/6 inhibitors in ER+ breast cancer.NPJ Precis Oncol. 2022 Sep 24;6(1):68. doi: 10.1038/s41698-022-00311-6. NPJ Precis Oncol. 2022. PMID: 36153348 Free PMC article.
-
Cyclin-dependent kinase inhibitors in brain cancer: current state and future directions.Cancer Drug Resist. 2020 Mar 19;3(1):48-62. doi: 10.20517/cdr.2019.105. eCollection 2020. Cancer Drug Resist. 2020. PMID: 35582046 Free PMC article. Review.
-
Diisothiocyanate-Derived Mercapturic Acids Are a Promising Partner for Combination Therapies in Glioblastoma.ACS Omega. 2022 Feb 8;7(7):5929-5936. doi: 10.1021/acsomega.1c06169. eCollection 2022 Feb 22. ACS Omega. 2022. PMID: 35224353 Free PMC article.
References
-
- Bastien JI, McNeill KA, Fine HA. (2015) Molecular characterizations of glioblastoma, targeted therapy, and clinical results to date. Cancer 121:502–516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
