Adrenergic Stimulation of DUSP1 Impairs Chemotherapy Response in Ovarian Cancer
- PMID: 26581245
- PMCID: PMC4818718
- DOI: 10.1158/1078-0432.CCR-15-1275
Adrenergic Stimulation of DUSP1 Impairs Chemotherapy Response in Ovarian Cancer
Abstract
Purpose: Chronic adrenergic activation has been shown to associate with adverse clinical outcomes in cancer patients, but the underlying mechanisms are not well understood. The focus of the current study was to determine the functional and biologic effects of adrenergic pathways on response to chemotherapy in the context of ovarian cancer.
Experimental design: Increased DUSP1 production by sympathetic nervous system mediators (e.g., norepinephrine) was analyzed by real-time quantitative RT-PCR and by Western blotting. In vitro chemotherapy-induced cell apoptosis was examined by flow cytometry. For in vivo therapy, a well-characterized model of chronic stress was used.
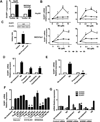
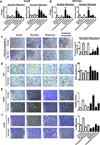
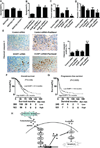
Results: Catecholamines significantly inhibited paclitaxel- and cisplatin-induced apoptosis in ovarian cancer cells. Genomic analyses of cells treated with norepinephrine identified DUSP1 as a potential mediator. DUSP1 overexpression resulted in reduced paclitaxel-induced apoptosis in ovarian cancer cells compared with control; conversely, DUSP1 gene silencing resulted in increased apoptosis compared with control cells. DUSP1 gene silencing in vivo significantly enhanced response to paclitaxel and increased apoptosis. In vitro analyses indicated that norepinephrine-induced DUSP1 gene expression was mediated through ADRB2 activation of cAMP-PLC-PKC-CREB signaling, which inhibits JNK-mediated phosphorylation of c-Jun and protects ovarian cancer cells from apoptosis. Moreover, analysis of The Cancer Genome Atlas data showed that increased DUSP1 expression was associated with decreased overall (P= 0.049) and progression-free (P= 0.0005) survival.
Conclusions: These findings provide a new understanding of the mechanisms by which adrenergic pathways can impair response to chemotherapy and have implications for cancer management.
©2015 American Association for Cancer Research.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures



Similar articles
-
DUSP1 is a novel target for enhancing pancreatic cancer cell sensitivity to gemcitabine.PLoS One. 2014 Jan 7;9(1):e84982. doi: 10.1371/journal.pone.0084982. eCollection 2014. PLoS One. 2014. PMID: 24409315 Free PMC article.
-
DUSP1 induces paclitaxel resistance through the regulation of p-glycoprotein expression in human ovarian cancer cells.Biochem Biophys Res Commun. 2016 Sep 9;478(1):403-409. doi: 10.1016/j.bbrc.2016.07.035. Epub 2016 Jul 13. Biochem Biophys Res Commun. 2016. PMID: 27422607
-
ICER evokes Dusp1-p38 pathway enhancing chemotherapy sensitivity in myeloid leukemia.Clin Cancer Res. 2011 Feb 15;17(4):742-52. doi: 10.1158/1078-0432.CCR-10-0886. Clin Cancer Res. 2011. PMID: 21325296
-
Role of DUSP1/MKP1 in tumorigenesis, tumor progression and therapy.Cancer Med. 2016 Aug;5(8):2061-8. doi: 10.1002/cam4.772. Epub 2016 May 26. Cancer Med. 2016. PMID: 27227569 Free PMC article. Review.
-
Dual-specificity phosphatases: therapeutic targets in cancer therapy resistance.J Cancer Res Clin Oncol. 2022 Jan;148(1):57-70. doi: 10.1007/s00432-021-03874-2. Epub 2022 Jan 4. J Cancer Res Clin Oncol. 2022. PMID: 34981193 Review.
Cited by
-
Impact of a natural disaster on access to care and biopsychosocial outcomes among Hispanic/Latino cancer survivors.Sci Rep. 2020 Jun 25;10(1):10376. doi: 10.1038/s41598-020-66628-z. Sci Rep. 2020. PMID: 32587352 Free PMC article.
-
Zoledronic Acid Abrogates Restraint Stress-Induced Macrophage Infiltration, PDGF-AA Expression, and Ovarian Cancer Growth.Cancers (Basel). 2020 Sep 18;12(9):2671. doi: 10.3390/cancers12092671. Cancers (Basel). 2020. PMID: 32962103 Free PMC article.
-
Pathogenesis and therapeutic strategies for cancer-related depression.Am J Cancer Res. 2024 Sep 15;14(9):4197-4217. doi: 10.62347/WVVG5364. eCollection 2024. Am J Cancer Res. 2024. PMID: 39417166 Free PMC article. Review.
-
The Role of Psychologic Stress in Cancer Initiation: Clinical Relevance and Potential Molecular Mechanisms.Cancer Res. 2021 Oct 15;81(20):5131-5140. doi: 10.1158/0008-5472.CAN-21-0684. Epub 2021 Jul 15. Cancer Res. 2021. PMID: 34266894 Free PMC article. Review.
-
Multiple types of distress are prospectively associated with increased risk of ovarian cancer.Cancer Med. 2023 Jul;12(14):15404-15413. doi: 10.1002/cam4.6125. Epub 2023 Jun 16. Cancer Med. 2023. PMID: 37326414 Free PMC article.
References
-
- Su F, Ouyang N, Zhu P, Ouyang N, Jia W, Gong C, et al. Psychological stress induces chemoresistance in breast cancer by upregulating mdr1. Biochemical and biophysical research communications. 2005;329:888–897. - PubMed
-
- Flint MS, Kim G, Hood BL, Bateman NW, Stewart NA, Conrads TP. Stress hormones mediate drug resistance to paclitaxel in human breast cancer cells through a CDK-1-dependent pathway. Psychoneuroendocrinology. 2009;34:1533–1541. - PubMed
-
- Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nature medicine. 2006;12:939–944. - PubMed
-
- Wu W, Pew T, Zou M, Pang D, Conzen SD. Glucocorticoid receptor-induced MAPK phosphatase-1 (MPK-1) expression inhibits paclitaxel-associated MAPK activation and contributes to breast cancer cell survival. J Biol Chem. 2005;280:4117–4124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 CA101642/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- R01 CA177909/CA/NCI NIH HHS/United States
- R01 CA109298/CA/NCI NIH HHS/United States
- P50 CA098258/CA/NCI NIH HHS/United States
- CA104825/CA/NCI NIH HHS/United States
- R01 CA193249/CA/NCI NIH HHS/United States
- P50 CA083639/CA/NCI NIH HHS/United States
- CA 109298/CA/NCI NIH HHS/United States
- R01 CA140933/CA/NCI NIH HHS/United States
- CA140933/CA/NCI NIH HHS/United States
- CA098258/CA/NCI NIH HHS/United States
- R01 CA104825/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

