Anatomical localization of Cav3.1 calcium channels and electrophysiological effects of T-type calcium channel blockade in the motor thalamus of MPTP-treated monkeys
- PMID: 26538609
- PMCID: PMC4760490
- DOI: 10.1152/jn.00858.2015
Anatomical localization of Cav3.1 calcium channels and electrophysiological effects of T-type calcium channel blockade in the motor thalamus of MPTP-treated monkeys
Abstract
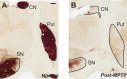
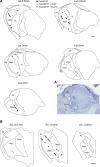
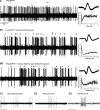
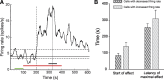
Conventional anti-Parkinsonian dopamine replacement therapy is often complicated by side effects that limit the use of these medications. There is a continuing need to develop nondopaminergic approaches to treat Parkinsonism. One such approach is to use medications that normalize dopamine depletion-related firing abnormalities in the basal ganglia-thalamocortical circuitry. In this study, we assessed the potential of a specific T-type calcium channel blocker (ML218) to eliminate pathologic burst patterns of firing in the basal ganglia-receiving territory of the motor thalamus in Parkinsonian monkeys. We also carried out an anatomical study, demonstrating that the immunoreactivity for T-type calcium channels is strongly expressed in the motor thalamus in normal and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated monkeys. At the electron microscopic level, dendrites accounted for >90% of all tissue elements that were immunoreactive for voltage-gated calcium channel, type 3.2-containing T-type calcium channels in normal and Parkinsonian monkeys. Subsequent in vivo electrophysiologic studies in awake MPTP-treated Parkinsonian monkeys demonstrated that intrathalamic microinjections of ML218 (0.5 μl of a 2.5-mM solution, injected at 0.1-0.2 μl/min) partially normalized the thalamic activity by reducing the proportion of rebound bursts and increasing the proportion of spikes in non-rebound bursts. The drug also attenuated oscillatory activity in the 3-13-Hz frequency range and increased gamma frequency oscillations. However, ML218 did not normalize Parkinsonism-related changes in firing rates and oscillatory activity in the beta frequency range. Whereas the described changes are promising, a more complete assessment of the cellular and behavioral effects of ML218 (or similar drugs) is needed for a full appraisal of their anti-Parkinsonian potential.
Keywords: Parkinsonism; T-type calcium channel; nondopaminergic therapy; rebound burst.
Copyright © 2016 the American Physiological Society.
Figures



Similar articles
-
Lack of Antiparkinsonian Effects of Systemic Injections of the Specific T-Type Calcium Channel Blocker ML218 in MPTP-Treated Monkeys.ACS Chem Neurosci. 2016 Nov 16;7(11):1543-1551. doi: 10.1021/acschemneuro.6b00186. Epub 2016 Sep 20. ACS Chem Neurosci. 2016. PMID: 27596273 Free PMC article.
-
Sub-synaptic localization of Cav3.1 T-type calcium channels in the thalamus of normal and parkinsonian monkeys.Brain Struct Funct. 2017 Mar;222(2):735-748. doi: 10.1007/s00429-016-1242-9. Epub 2016 Jun 2. Brain Struct Funct. 2017. PMID: 27255751 Free PMC article.
-
Effects of high-frequency stimulation of the internal pallidal segment on neuronal activity in the thalamus in parkinsonian monkeys.J Neurophysiol. 2016 Dec 1;116(6):2869-2881. doi: 10.1152/jn.00104.2016. Epub 2016 Sep 28. J Neurophysiol. 2016. PMID: 27683881 Free PMC article.
-
The role of T-channels in the generation of thalamocortical rhythms.CNS Neurol Disord Drug Targets. 2006 Dec;5(6):571-85. doi: 10.2174/187152706779025526. CNS Neurol Disord Drug Targets. 2006. PMID: 17168743 Review.
-
Changing views of the pathophysiology of Parkinsonism.Mov Disord. 2019 Aug;34(8):1130-1143. doi: 10.1002/mds.27741. Epub 2019 Jun 19. Mov Disord. 2019. PMID: 31216379 Review.
Cited by
-
Structural plasticity of GABAergic and glutamatergic networks in the motor thalamus of parkinsonian monkeys.J Comp Neurol. 2020 Jun;528(8):1436-1456. doi: 10.1002/cne.24834. Epub 2019 Dec 16. J Comp Neurol. 2020. PMID: 31808567 Free PMC article.
-
Cortical Serotonergic and Catecholaminergic Denervation in MPTP-Treated Parkinsonian Monkeys.Cereb Cortex. 2022 Apr 20;32(9):1804-1822. doi: 10.1093/cercor/bhab313. Cereb Cortex. 2022. PMID: 34519330 Free PMC article.
-
Simple models including energy and spike constraints reproduce complex activity patterns and metabolic disruptions.PLoS Comput Biol. 2020 Dec 21;16(12):e1008503. doi: 10.1371/journal.pcbi.1008503. eCollection 2020 Dec. PLoS Comput Biol. 2020. PMID: 33347433 Free PMC article.
-
Changes in Excitability Properties of Ventromedial Motor Thalamic Neurons in 6-OHDA Lesioned Mice.eNeuro. 2021 Feb 24;8(1):ENEURO.0436-20.2021. doi: 10.1523/ENEURO.0436-20.2021. Print 2021 Jan-Feb. eNeuro. 2021. PMID: 33509950 Free PMC article.
-
Interactions Between Motor Thalamic Field Potentials and Single-Unit Spiking Are Correlated With Behavior in Rats.Front Neural Circuits. 2020 Aug 13;14:52. doi: 10.3389/fncir.2020.00052. eCollection 2020. Front Neural Circuits. 2020. PMID: 32922268 Free PMC article.
References
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci 12: 366–375, 1989. - PubMed
-
- Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of Parkinsonism. J Neurophysiol 72: 507–520, 1994. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

