Phenotypic differences in nebivolol metabolism and bioavailability in healthy volunteers
- PMID: 26528073
- PMCID: PMC4576779
- DOI: 10.15386/cjmed-395
Phenotypic differences in nebivolol metabolism and bioavailability in healthy volunteers
Erratum in
-
Erratum.Med Pharm Rep. 2020 Oct;93(4):435. Epub 2020 Oct 25. Med Pharm Rep. 2020. PMID: 33225273 Free PMC article.
Abstract
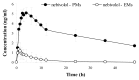
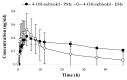
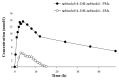
Introduction: Nebivolol, a third-generation β-blocker, is subject to extensive first-pass metabolism and produces active β-blocking hydroxylated metabolites, like 4-OH-nebivolol. It is primarily a substrate of CYP2D6, a metabolic pathway that is under polymorphic genetic regulation. The objective of this study was to assess the metabolizer phenotype and to evaluate the interphenotype bioavailability and metabolism of nebivolol.
Material and methods: Forty-three healthy volunteers were included in this open-label, non-randomized clinical trial and each volunteer received a single dose of 5 mg nebivolol. Non-compartmental pharmacokinetic analysis was performed to determine the pharmacokinetic parameters of nebivolol and its active metabolite. The phenotypic distribution was assessed based on the AUC (aria under the curve) metabolic ratio of nebivolol/4-OH-nebivolol and statistical analysis. An interphenotype comparison of nebivolol metabolism and bioavailability was performed based on the pharmacokinetic parameters of nebivolol and its active metabolite.
Results: Nebivolol/4-OH-nebivolol AUC metabolic ratios were not characterized by a standard normal distribution. The unique distribution emphasized the existence of two groups and the 43 healthy volunteers were classified as follows: poor metabolizers (PMs)=3, extensive metabolizers (EMs)=40. The phenotype had a marked impact on nebivolol metabolism. The exposure to nebivolol was 15-fold greater for PMs in comparison to EMs.
Conclusion: 40 EMs and 3 PMs were differentiated by using the pharmacokinetic parameters of nebivolol and its active metabolite. The study highlighted the existence of interphenotype differences regarding nebivolol metabolism and bioavailability.
Keywords: CYP2D6; nebivolol; pharmacokinetics; phenotype.
Figures

Similar articles
-
The influence of CYP2D6 phenotype on the clinical response of nebivolol in patients with essential hypertension.Br J Clin Pharmacol. 2007 May;63(5):575-82. doi: 10.1111/j.1365-2125.2006.02796.x. Epub 2006 Nov 10. Br J Clin Pharmacol. 2007. PMID: 17094780 Free PMC article. Clinical Trial.
-
A pharmacokinetic drug interaction study between nebivolol and paroxetine in healthy volunteers.J Clin Pharm Ther. 2014 Oct;39(5):535-40. doi: 10.1111/jcpt.12180. Epub 2014 May 21. J Clin Pharm Ther. 2014. PMID: 24845234 Clinical Trial.
-
Effect of CYP2D6 Poor Metabolizer Phenotype on Stereoselective Nebivolol Pharmacokinetics.Drug Metab Lett. 2018;12(1):68-70. doi: 10.2174/1872312812666180420104945. Drug Metab Lett. 2018. PMID: 29676238
-
Polymorphism of human cytochrome P450 2D6 and its clinical significance: Part I.Clin Pharmacokinet. 2009;48(11):689-723. doi: 10.2165/11318030-000000000-00000. Clin Pharmacokinet. 2009. PMID: 19817501 Review.
-
Clinical pharmacokinetics of nebivolol: a systematic review.Drug Metab Rev. 2023 Nov;55(4):428-440. doi: 10.1080/03602532.2023.2271195. Epub 2023 Oct 28. Drug Metab Rev. 2023. PMID: 37849071 Review.
Cited by
-
Therapeutic Properties of Highly Selective β-blockers With or Without Additional Vasodilator Properties: Focus on Bisoprolol and Nebivolol in Patients With Cardiovascular Disease.Cardiovasc Drugs Ther. 2022 Oct;36(5):959-971. doi: 10.1007/s10557-021-07205-y. Epub 2021 Jun 9. Cardiovasc Drugs Ther. 2022. PMID: 34106365 Free PMC article. Review.
-
Protective Effect of Nebivolol against Oxidative Stress Induced by Aristolochic Acids in Endothelial Cells.Toxins (Basel). 2022 Feb 10;14(2):132. doi: 10.3390/toxins14020132. Toxins (Basel). 2022. PMID: 35202159 Free PMC article.
-
Physiologically-Based Pharmacokinetic Models of CYP2D6 Substrate and Inhibitors Nebivolol, Cinacalcet and Mirabegron to Simulate Drug-Drug Interactions.Eur J Drug Metab Pharmacokinet. 2022 Sep;47(5):699-710. doi: 10.1007/s13318-022-00775-8. Epub 2022 Jul 15. Eur J Drug Metab Pharmacokinet. 2022. PMID: 35840839
-
Pharmacogenetic factors affecting β-blocker metabolism and response.Expert Opin Drug Metab Toxicol. 2020 Oct;16(10):953-964. doi: 10.1080/17425255.2020.1803279. Epub 2020 Sep 9. Expert Opin Drug Metab Toxicol. 2020. PMID: 32726152 Free PMC article. Review.
-
Identification of the New Metabolite of Nebivolol Using Liquid Chromatography Coupled with High-Resolution Mass Spectrometry and Chemometrics.Molecules. 2022 Jan 24;27(3):763. doi: 10.3390/molecules27030763. Molecules. 2022. PMID: 35164027 Free PMC article.
References
-
- Baldwin CM, Keam SJ. Nebivolol in the treatment of hypertension in the US. Am J Cardiovasc Drugs. 2009;9(4):253–260. - PubMed
-
- Moen MD, Wagstaff AJ. Nebivolol: A Review of its use in the management of hypertension and chronic heart failure. Drugs. 2006;66(10):1389–1409. - PubMed
-
- Zanchetti A. Clinical pharmacodynamics of nebivolol: new evidence of nitric oxide-mediated vasodilating activity and peculiar haemodynamic properties in hypertensive patients. Blood Press Suppl. 2004;1:17–32. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
