Antithymocyte globulin treatment at the time of transplantation impairs donor hematopoietic stem cell engraftment
- PMID: 26499257
- PMCID: PMC5423086
- DOI: 10.1038/cmi.2015.92
Antithymocyte globulin treatment at the time of transplantation impairs donor hematopoietic stem cell engraftment
Abstract
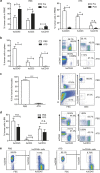
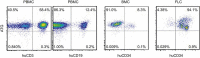
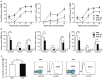
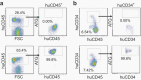
Antithymocyte globulin (ATG) is often included in the conditioning regimen to prevent graft vs. host disease in allogeneic hematopoietic stem cell (HSC) transplantation. However, because ATG contains antibodies targeting a wide range of antigens on human cells, its potential off-target effects remain a concern. Here, we explored this question in humanized mice that permit the analysis of human cell depletion in tissues. We showed that ATG binds to almost all lineages of human hematopoietic cells including HSCs, and accordingly it is capable of depleting almost all human hematopoietic cells. Interestingly, the efficacy of ATG was highly variable depending on the tissue of residence, with human cells in bone marrow significantly less susceptible than those in the blood and spleen. Recovery of multilineage human lymphohematopoietic reconstitution in humanized mice that received ATG 3 weeks after HSC transplantation indicates that ATG had a minimal effect on human HSCs that have settled in bone marrow niches. However, efficient human HSC depletion and engraftment failure were seen in mice receiving ATG at the time of transplantation. Our data indicate that the efficacy of ATG is tissue-dependent, and suggest a potential risk of impairing donor hematopoietic engraftment when ATG is used in preparative conditioning regimens.
Figures


Similar articles
-
Effect of ATG-F on B-cell reconstitution after hematopoietic stem cell transplantation.Eur J Haematol. 2015 Dec;95(6):514-23. doi: 10.1111/ejh.12524. Epub 2015 Mar 13. Eur J Haematol. 2015. PMID: 25677646
-
Donor hematopoiesis in mice following total lymphoid irradiation requires host T-regulatory cells for durable engraftment.Blood. 2014 May 1;123(18):2882-92. doi: 10.1182/blood-2013-10-530212. Epub 2014 Mar 3. Blood. 2014. PMID: 24591203 Free PMC article.
-
Antithymocyte globulin in allogeneic hematopoietic cell transplantation: benefits and limitations.Immunotherapy. 2016;8(4):435-47. doi: 10.2217/imt.15.128. Immunotherapy. 2016. PMID: 26973125 Review.
-
What role is there for antithymocyte globulin in allogeneic nonmyeloablative canine hematopoietic cell transplantation?Biol Blood Marrow Transplant. 2005 May;11(5):335-44. doi: 10.1016/j.bbmt.2005.01.001. Biol Blood Marrow Transplant. 2005. PMID: 15846286 Free PMC article.
-
Rabbit anti-T cell globulin in allogeneic hematopoietic cell transplantation.Biol Blood Marrow Transplant. 2015 Jun;21(6):959-70. doi: 10.1016/j.bbmt.2014.11.676. Epub 2014 Dec 5. Biol Blood Marrow Transplant. 2015. PMID: 25482864 Review.
Cited by
-
Distinct Immune Homeostasis Remodeling Patterns after HLA-Matched and Haploidentical Transplantation.Adv Sci (Weinh). 2024 Oct;11(39):e2400544. doi: 10.1002/advs.202400544. Epub 2024 Sep 3. Adv Sci (Weinh). 2024. PMID: 39225336 Free PMC article.
-
LIS1, a glyco-humanized swine polyclonal anti-lymphocyte globulin, as a novel induction treatment in solid organ transplantation.Front Immunol. 2023 Feb 16;14:1137629. doi: 10.3389/fimmu.2023.1137629. eCollection 2023. Front Immunol. 2023. PMID: 36875084 Free PMC article.
-
Non-genotoxic conditioning facilitates hematopoietic stem cell gene therapy for hemophilia A using bioengineered factor VIII.Mol Ther Methods Clin Dev. 2021 May 5;21:710-727. doi: 10.1016/j.omtm.2021.04.016. eCollection 2021 Jun 11. Mol Ther Methods Clin Dev. 2021. PMID: 34141826 Free PMC article.
-
Peripheral host T cells survive hematopoietic stem cell transplantation and promote graft-versus-host disease.J Clin Invest. 2020 Sep 1;130(9):4624-4636. doi: 10.1172/JCI129965. J Clin Invest. 2020. PMID: 32516138 Free PMC article. Clinical Trial.
-
Complement Depletion Improves Human Red Blood Cell Reconstitution in Immunodeficient Mice.Stem Cell Reports. 2017 Oct 10;9(4):1034-1042. doi: 10.1016/j.stemcr.2017.08.018. Epub 2017 Sep 28. Stem Cell Reports. 2017. PMID: 28966117 Free PMC article.
References
-
- Brennan DC, Schnitzler MA. Long-term results of rabbit antithymocyte globulin and basiliximab induction. N Engl J Med 2008; 359: 1736–1738. - PubMed
-
- Hardinger KL, Schnitzler MA, Miller B, Lowell JA, Shenoy S, Koch MJ et al. Five-year follow up of thymoglobulin versus ATGAM induction in adult renal transplantation. Transplantation 2004; 78: 136–141. - PubMed
-
- Cantarovich D, Rostaing L, Kamar N, Ducloux D, Saint-Hillier Y, Mourad G et al. Early corticosteroid avoidance in kidney transplant recipients receiving ATG-F induction: 5-year actual results of a prospective and randomized study. Am J Transplant 2014; 14: 2556–2564. - PubMed
-
- Lindemans CA, Chiesa R, Amrolia PJ, Rao K, Nikolajeva O, de Wildt A et al. Impact of thymoglobulin prior to pediatric unrelated umbilical cord blood transplantation on immune reconstitution and clinical outcome. Blood 2014; 123: 126–132. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

