Macrophage Blockade Using CSF1R Inhibitors Reverses the Vascular Leakage Underlying Malignant Ascites in Late-Stage Epithelial Ovarian Cancer
- PMID: 26471360
- PMCID: PMC4675660
- DOI: 10.1158/0008-5472.CAN-14-3373
Macrophage Blockade Using CSF1R Inhibitors Reverses the Vascular Leakage Underlying Malignant Ascites in Late-Stage Epithelial Ovarian Cancer
Abstract
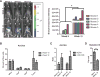
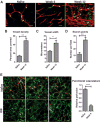
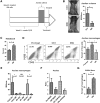
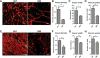
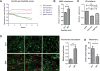
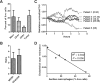
Malignant ascites is a common complication in the late stages of epithelial ovarian cancer (EOC) that greatly diminishes the quality of life of patients. Malignant ascites is a known consequence of vascular dysfunction, but current approved treatments are not effective in preventing fluid accumulation. In this study, we investigated an alternative strategy of targeting macrophage functions to reverse the vascular pathology of malignant ascites using fluid from human patients and an immunocompetent murine model (ID8) of EOC that mirrors human disease by developing progressive vascular disorganization and leakiness culminating in massive ascites. We demonstrate that the macrophage content in ascites fluid from human patients and the ID8 model directly correlates with vascular permeability. To further substantiate macrophages' role in the pathogenesis of malignant ascites, we blocked macrophage function in ID8 mice using a colony-stimulating factor 1 receptor kinase inhibitor (GW2580). Administration of GW2580 in the late stages of disease resulted in reduced infiltration of protumorigenic (M2) macrophages and dramatically decreased ascites volume. Moreover, the disorganized peritoneal vasculature became normalized and sera from GW2580-treated ascites protected against endothelial permeability. Therefore, our findings suggest that macrophage-targeted treatment may be a promising strategy toward a safe and effective means to control malignant ascites of EOC.
©2015 American Association for Cancer Research.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures
Similar articles
-
Modification of the Tumor Microenvironment in KRAS or c-MYC-Induced Ovarian Cancer-Associated Peritonitis.PLoS One. 2016 Aug 2;11(8):e0160330. doi: 10.1371/journal.pone.0160330. eCollection 2016. PLoS One. 2016. PMID: 27483433 Free PMC article.
-
Inhibition of colony-stimulating-factor-1 signaling in vivo with the orally bioavailable cFMS kinase inhibitor GW2580.Proc Natl Acad Sci U S A. 2005 Nov 1;102(44):16078-83. doi: 10.1073/pnas.0502000102. Epub 2005 Oct 25. Proc Natl Acad Sci U S A. 2005. PMID: 16249345 Free PMC article.
-
Systemic and Cardiac Depletion of M2 Macrophage through CSF-1R Signaling Inhibition Alters Cardiac Function Post Myocardial Infarction.PLoS One. 2015 Sep 25;10(9):e0137515. doi: 10.1371/journal.pone.0137515. eCollection 2015. PLoS One. 2015. PMID: 26407006 Free PMC article.
-
Metastatic Voyage of Ovarian Cancer Cells in Ascites with the Assistance of Various Cellular Components.Int J Mol Sci. 2022 Apr 15;23(8):4383. doi: 10.3390/ijms23084383. Int J Mol Sci. 2022. PMID: 35457198 Free PMC article. Review.
-
Suppression of epithelial ovarian cancer invasion into the omentum by 1α,25-dihydroxyvitamin D3 and its receptor.J Steroid Biochem Mol Biol. 2015 Apr;148:138-47. doi: 10.1016/j.jsbmb.2014.11.005. Epub 2014 Nov 6. J Steroid Biochem Mol Biol. 2015. PMID: 25448740 Free PMC article. Review.
Cited by
-
Perivascular Macrophages Limit Permeability.Arterioscler Thromb Vasc Biol. 2016 Nov;36(11):2203-2212. doi: 10.1161/ATVBAHA.116.307592. Epub 2016 Sep 15. Arterioscler Thromb Vasc Biol. 2016. PMID: 27634833 Free PMC article.
-
Preclinical and Clinical Immunotherapeutic Strategies in Epithelial Ovarian Cancer.Cancers (Basel). 2020 Jul 2;12(7):1761. doi: 10.3390/cancers12071761. Cancers (Basel). 2020. PMID: 32630708 Free PMC article. Review.
-
Crosstalk of Immune Cells and Platelets in an Ovarian Cancer Microenvironment and Their Prognostic Significance.Int J Mol Sci. 2023 May 25;24(11):9279. doi: 10.3390/ijms24119279. Int J Mol Sci. 2023. PMID: 37298230 Free PMC article. Review.
-
FGFR inhibitors: Effects on cancer cells, tumor microenvironment and whole-body homeostasis (Review).Int J Mol Med. 2016 Jul;38(1):3-15. doi: 10.3892/ijmm.2016.2620. Epub 2016 May 31. Int J Mol Med. 2016. PMID: 27245147 Free PMC article. Review.
-
ALOX5AP Predicts Poor Prognosis by Enhancing M2 Macrophages Polarization and Immunosuppression in Serous Ovarian Cancer Microenvironment.Front Oncol. 2021 May 19;11:675104. doi: 10.3389/fonc.2021.675104. eCollection 2021. Front Oncol. 2021. PMID: 34094977 Free PMC article.
References
-
- Chung M, Kozuch P. Treatment of malignant ascites. Curr Treat Options Oncol. 2008;9:215–33. - PubMed
-
- Kobold S, Hegewisch-Becker S, Oechsle K, Jordan K, Bokemeyer C, Atanackovic D. Intraperitoneal VEGF inhibition using bevacizumab: a potential approach for the symptomatic treatment of malignant ascites? Oncologist. 2009;14:1242–51. - PubMed
-
- Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

